Ethyl octanoate
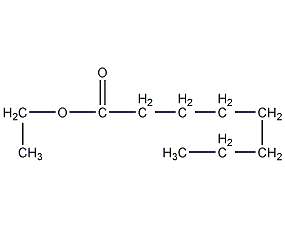

Structural formula
| Business number | 02T4 |
|---|---|
| Molecular formula | C10H20O2 |
| Molecular weight | 172.26 |
| label |
Ethyl Caprylate, Ethyl n-octanoate, Ethyl subcaprylate, Ethyl caprylate, Ethyl octoate, Caprylic acid ethyl ester |
Numbering system
CAS number:106-32-1
MDL number:MFCD00009552
EINECS number:203-385-5
RTECS number:RH0680000
BRN number:1754470
PubChem ID:None
Physical property data
1. Properties: colorless liquid with fruity aroma, pineapple, apple-like aroma and brandy aroma.
2. Density (g/mL, 25℃): 0.866
3. Relative density (20℃, 4℃): 0.8667
4. Melting point (ºC): -43.1
5. Boiling point (ºC, normal pressure): 206~208
6. Relative density (25℃, 4℃): 0.8624
7. Refractive index (n20D): 1.4175
8. Flash point (ºC): 75
9. Refractive index at room temperature (n25): 1.4158
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 25ºC): 0.02
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol ): Undetermined
14. Critical temperature (ºC): 375.85
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol /water) logarithmic value of the distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V) : Undetermined
19. Solubility: Insoluble in water and propylene glycol, easily soluble in ethanol, ether and chloroform.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 500mg/24HREACTION SEVERITY, moderate reaction; 2. Acute toxicity: rat oral LD50: 2596mg/kg
Ecological data
This substance may be harmful to the environment and it is recommended not to let it enter the environment.
Molecular structure data
1. Molar refractive index: 50.15
2. Molar volume (cm3/mol): 197.0
3. Isotonic specific volume (90.2K ): 454.7
4. Surface tension (dyne/cm): 28.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 19.88
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 110
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants, reducing agents, acids, and alkalis.
2. Found in flue-cured tobacco leaves.
3. Naturally found in some fruits and brandy.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the esterification of octanoic acid and ethanol. Add octanoic acid, ethanol and a small amount of concentrated sulfuric acid into the reaction pot, heat and reflux for several hours, then neutralize with sodium hydroxide solution, and then distill it.
2. Tobacco: FC, 18.
Purpose
1. Used as food flavoring agent and spice preparation.
2. Mainly used to prepare edible flavors such as apricots, apples, and grapes, as well as wine flavors such as brandy and wine.