Ethyl pyruvate

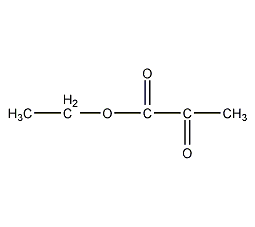
Structural formula
| Business number | 06HY |
|---|---|
| Molecular formula | C5H8O3 |
| Molecular weight | 116 |
| label |
2-Oxopropionic acid ethyl ester, Ethyl pyruvate |
Numbering system
CAS number:617-35-6
MDL number:MFCD00009123
EINECS number:210-511-2
RTECS number:None
BRN number:1071466
PubChem number:24901103
Physical property data
Physical property data: 1. Appearance: colorless liquid
2. Melting point -50℃
3. Boiling point 155℃, 147.5℃ (100kPa), 69-71℃ (5.7kPa), 48.5℃ ( 0.67kPa)
4. Relative density 1.0596 (15.5/4℃)
5. Refractive index at room temperature (n20): 1.4052
6. Flash point 45℃
7. Solubility: soluble in ethanol, ether, acetone, slightly soluble in water
8. Relative density (20℃, 4℃ ): 1.059615.6
Toxicological data
None yet
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 27.01
2. Molar volume (cm3/mol): 111.6
3. Isotonic specific volume (90.2K): 260.8
4. Surface tension (dyne/cm): 29.7
5. Polarizability (10-24cm3): 10.71
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 43.4
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 106
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Properties: colorless liquid.
Storage method
Storage:
Seal the container and store it in a sealed main container in a cool, dry place.
Synthesis method
1. Preparation method: Obtained from oxidation of ethyl lactate with potassium permanganate.
2. Preparation method:

In a reaction bottle equipped with a stirrer, thermometer, and dropping funnel, add 130 mL of saturated magnesium sulfate solution and 500 mL of petroleum ether at 30 to 60°C. Ethyl lactate (2) 50g (0.42mol), 20g (0.13mol) potassium dihydrogen phosphate. Cool to 15°C in an ice water bath, add 55g potassium permanganate powder (0.35mol) in batches while stirring, pay attention to the addition speed, wait After the reaction of the previous batch is complete, add the next batch and control the reaction temperature at about 15°C. After the reaction is completed, let it stand and separate the layers. Separate the organic layer, and extract the lower slurry three times with petroleum ether. Combine the organic layers and recover them by distillation After removing petroleum ether, the residue was washed twice with saturated calcium chloride solution, 10 mL each time. Distilled under reduced pressure and collected the fraction at 56~57°C/2.67KPa to obtain 25~27g of ethyl pyruvate (1), yield 51 %~54%.[1]
Purpose
3. Usage: used in organic synthesis.