Isooctene Isooctene
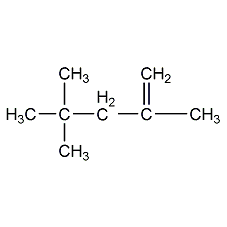

Structural formula
| Business number | 02V6 |
|---|---|
| Molecular formula | C8H16 |
| Molecular weight | 112.21 |
| label |
butylene, 2,4,4-Trimethyl-1-pentene, diisobutylene, 2,4,4-Trimethyl-1-pentene |
Numbering system
CAS number:107-39-1
MDL number:MFCD00008855
EINECS number:203-486-4
RTECS number:SB2717300
BRN number:1098309
PubChem number:24900479
Physical property data
1. Properties: colorless, transparent, volatile liquid [1]
2. Melting point (℃): -105[2]
3. Boiling point (℃): 112~113[3]
4. Relative density (water=1): 0.72 (15.5℃)[4]
5. Saturated vapor pressure (kPa): 3.35 (25℃)[5]
6. Critical pressure (MPa): 2.6[6]
7. Octanol/water partition coefficient: 4.06[7]
8 .Flash point (℃): 3[8]
9. Ignition temperature (℃): 274[9]
10. Explosion upper limit (%): 5.5[10]
11. Explosion lower limit (%): 0.9[11]
12. Solubility: No data yet
13. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5324.18
14. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -110.1
15. Solubility parameter (J·cm-3)0.5: 14.522
16. van der Waals area (cm2·mol-1): 1.230×1010
17. van der Waals volume (cm3·mol-1): 85.190 p>
18. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -5288.41
19. Liquid phase standard claimed heat (enthalpy) ( kJ·mol-1): -145.9
20. Saturated vapor pressure (kPa, 21ºC): 13.6
21. Liquid phase standard entropy (J ·mol-1·K-1): 309.0
22. Liquid phase standard formation free energy (kJ·mol-1): 87.1
23. Liquid phase standard hot melt (J·mol-1·K-1): 238.5
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[12] This substance may be harmful to the environment and is harmful to the environment. Bodies of water should be given special attention.
Molecular structure data
1. Molar refractive index: 38.97
2. Molar volume (cm3/mol): 154.0
3. Isotonic specific volume (90.2K ): 328.9
4. Surface tension (dyne/cm): 20.7
5. Polarizability (10-24cm3): 15.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 82.7
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[13] Stable
2. Incompatible substances[14] Strong oxidants, acids, halogenated hydrocarbons, halogens, etc.
3. Conditions to avoid contact[15] Heating
4. Aggregation hazards[16] Aggregation
Storage method
Storage Precautions[17] Usually products contain polymerization inhibitors. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, and peroxides, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It is industrially separated from the carbon four fractions in refineries or ethylene plants. The remaining part after the extraction of butadiene from the carbon four fraction is used as raw material, and diisobutylene is selectively absorbed with sulfuric acid. The sulfuric acid that has absorbed diisobutylene is then heated to separate and generate a polymer mainly composed of diisobutylene. This polymer is used After washing with sodium carbonate solution and refining by distillation, the above industrial product is obtained.
Purpose
1. This product is used as a tackifier for the preparation of synthetic rubber; various surfactants; modifier for phenolic resin and epoxy resin; ultraviolet absorber; polymerization inhibitor; polyvinyl chloride stabilizer; plasticizer It is also used to produce p-octylphenol, isononyl alcohol and other organic synthesis intermediates.
2. Used as solvent. [18]