Isopropyl formate
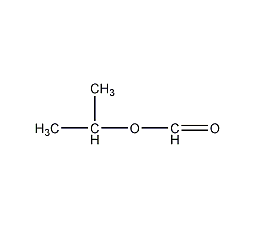

Structural formula
| Business number | 06S8 |
|---|---|
| Molecular formula | C4H8O2 |
| Molecular weight | 88.11 |
| label |
1-methylethyl formate, Isopropyl formate, Formic acid isopropyl ester, 1-Methylethyl Formate, aliphatic compounds |
Numbering system
CAS number:625-55-8
MDL number:MFCD00014127
EINECS number:210-901-2
RTECS number:LQ8750000
BRN number:1735844
PubChem number:24871257
Physical property data
1. Properties: colorless and transparent liquid.
2. Density (g/mL, 20℃): 0.8728
3. Density (g/mL, 25℃): 0.8716
4. Melting point (ºC): -93
5. Boiling point (ºC, normal pressure): 68.1
6. Refractive index (n20ºC): 1.3678
7. Refraction Rate (n25ºC): 1.366
8. Flash point (ºC, closed cup): -13
9. Viscosity (mPa·s, 20ºC): 0.522
10. Flash point (ºC): 485
11. Vapor pressure (kPa, 20ºC): 14.67
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
p>
13. Heat of combustion (KJ/mol): Undetermined
14. Relative steam density (g/mL, air=1): 3.03
15. Relative Evaporation rate (ether = 1): 2.7
16. Critical temperature (ºC): 261.85
17. Critical pressure (MPa): 3.95
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, solubility in water at 20°C is about 2%. Miscible with solvents such as ether and alcohol.
Toxicological data
1. Acute toxicity: Guinea pig (oral) LD50: 1,400 μg/kg
Since the LD50 of table salt is 3,000 mg/kg, the acute toxicity of BPA is the same as that of table salt.
2. High-concentration vapor has anesthetic properties.
Ecological data
Do not allow large quantities of products that are slightly harmful to water to come into contact with groundwater, waterways or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 22.47
2. Molar volume (cm3/mol): 98.6
3. Isotonic specific volume (90.2K ): 217.2
4. Surface tension (dyne/cm): 23.5
5. Polarizability (10-24cm3): 8.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 40.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Keep away from oxides and heat. It is easy to cause combustion when exposed to high heat, open flames and strong oxidants. Irritating.
2. Exist in smoke.
Storage method
Store in an airtight container in a cool, dry place. The storage place must be away from oxidants and protected from heat.
Synthesis method
It can be obtained directly by esterification method.
Purpose
Used in organic synthesis, solvent. Food spices. It can also be used as an antifungal agent and disinfectant. It can be used as a solvent for nitrocellulose and cellulose acetate.