Isotrigane Squalane
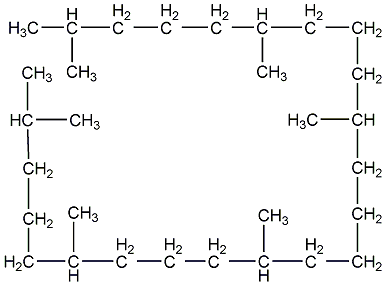

Structural formula
| Business number | 032M |
|---|---|
| Molecular formula | C30H62 |
| Molecular weight | 422.83 |
| label |
2,6,10,15,19,23-hexamethyltetracosane, squalane, Squalane; squalane;, 2,6,10,15,19,23-Hexamethyl-tetracosan, Perhydrosqualene, Lubricant |
Numbering system
CAS number:111-01-3
MDL number:MFCD00008953
EINECS number:203-825-6
RTECS number:XB6070000
BRN number:776019
PubChem number:24888405
Physical property data
1. Properties: Colorless and transparent oily viscous liquid.
2. Density (g/mL, 20℃): 0.8115
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -38
5. Boiling point (ºC, normal pressure): 350
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: 1.452
8. Flash point (ºC): 218
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC) : Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined Determined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ether, gasoline, petroleum ether, benzene, chloroform and oils , slightly soluble in methanol, ethanol, acetone and glacial acetic acid.
20. Freezing point (ºC): -38
Toxicological data
1. Irritation: Guinea pig transdermal: 100mg/24H Moderate irritation. Rabbit eye: 100mg/24H Slight irritation.
Ecological data
This substance is not harmful to water bodies.
Molecular structure data
1. Molar refractive index: 140.78
2. Molar volume (cm3/mol): 525.9
3. Isotonic specific volume (90.2K ): 1209.9
4. Surface tension (dyne/cm): 28.0
5. Dielectric constant (F/m): 2.12
6. Pole Chemical rate (10-24cm3): 55.81
Compute chemical data
1. Reference values for calculation of hydrophobic parameters(XlogP): 14.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds :21
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 30
p>
8. Surface charge: 0
9. Complexity: 308
10. Number of isotope atoms: 0
11. Determine the atomic position Number of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 4
13. Determined number of stereocenters of chemical bonds: 0
14. No Determine the number of stereocenters of chemical bonds: 0
15. The number of covalent bond units: 1
Properties and stability
It does not decompose under normal temperature and pressure. Contact with strong oxidants is prohibited.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together.
2. Equip with corresponding varieties and quantities of fire-fighting equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Squalane is produced by hydrogenation of squalene. Squalene is found in the livers of blue whales and sharks. It can be obtained by extraction and then refined to obtain squalene.
Purpose
1. Gas chromatography stationary solution (maximum operating temperature 150°C, solvent is toluene), separates and analyzes gaseous hydrocarbons and light fraction liquid hydrocarbons (C1~C8 ) lubricant. Transformer oil.
2. Squalane is less irritating to the skin and can make the skin soft. It itself or combined with other raw materials is very inert. , so it is an extremely stable oil raw material. Compared with mineral oil-based alkanes, it has a less greasy feel and has good skin permeability, lubricity and safety. It can be used as cream, lotion, lotion, etc. in cosmetics. Oil-based raw materials for lipstick and hair care products. The addition amount is 0.1%~50%.