m-methoxyphenol
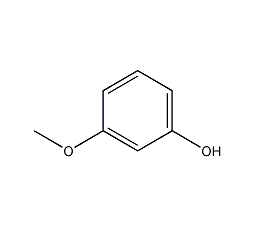

Structural formula
| Business number | 03Y2 |
|---|---|
| Molecular formula | C7H8O2 |
| Molecular weight | 124.14 |
| label |
3-methoxyphenol, 3-Methoxyphenol, 3-Hydroxyanisole, 3-Hydroxyanisole, 1-Hydroxy-3-anisole, 3-Hydroxyanisole, 1-Hydroxy-3-methyoxybenzene |
Numbering system
CAS number:150-19-6
MDL number:MFCD00002267
EINECS number:205-754-6
RTECS number:SL7524000
BRN number:1209898
PubChem number:24859737
Physical property data
1. Physical property data:
1. Properties: colorless, transparent to light yellow crystals, with a sweet aroma similar to phenol. Easily discolored when exposed to air.
2. Density (g/mL, 20℃): 1.145 3. Melting point (ºC): <-17 4. Boiling point (ºC, normal pressure): 243~246, 114ºC (666.5pa) 5. Refractive index (n20D): 1.551 6. Flash point (ºC): >1127. Solubility: Slightly soluble in water, soluble in ethanol and other organic solvents.
8. Relative density (20℃, 4℃): 1.115156
9. Relative density (25℃, 4℃): 1.052131 sup>
10. Refractive index at room temperature (n20): 1.5510
Toxicological data
2. Toxicological data:
1. Acute toxicity: Rat oral LD50: 597 mg/kg;
Rat dermal LD50: 682 mg/kg;
Mouse oral LD50: 312 mg/kg;
Mouse abdominal LD50: 320 mg/kg.
2. Inhalation toxicity: Rat LC50: 11500 mg/m3/4H;
Mouse LC50: 11500 mg/m3 /4H.
3. Reproductive toxicity: Rat oral TDLo: 333 mg/kg
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 34.81
2. Molar volume (cm3/mol): 111.8
3. Isotonic specific volume (90.2K): 278.9
4. Surface tension (dyne/cm): 38.6
5. Polarizability (10-24cm3): 13.80
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
p>
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complex Degree: 83
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters :0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Covalent bond units Quantity:1
Properties and stability
1. Stable under normal temperature and pressure.
2. Exist in oriental tobacco leaves and smoke.
3. Naturally found in rue oil, celery seed oil, tobacco leaf oil, orange leaf distillate and castoreum.
Storage method
Store sealed in a dry and cool place.
Synthesis method
1. Tobacco: OR, 26.
Purpose
1. Use sparingly in fragrances.
2. It is an important raw material for the synthesis of vanillin.