m-Nitroaniline m-Nitroaniline
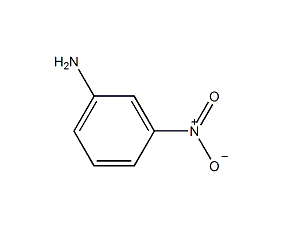
Structural formula
| Business number | 02F7 |
|---|---|
| Molecular formula | C6H6N2O2 |
| Molecular weight | 138 |
| label |
3-nitroaniline, 1-amino-3-nitrobenzene, 3-Nitroaniline, 3-Nitrobenzenamine, 1-Amino-3-nitrobenzene, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:99-09-2
MDL number:MFCD00007782
EINECS number:202-729-1
RTECS number:BY6825000
BRN number:636962
PubChem number:24869615
Physical property data
1.Characteristics: bright yellow needle-like crystals. [1]
2. Melting point (℃): 112~114[2]
3. Boiling point (℃) : 306 (decomposition) [3]
4. Relative density (water = 1): 1.42[4]
5. Saturated vapor pressure (kPa): 0.13 (119.3℃)[5]
6. Heat of combustion (kJ/mol): -3198.5[6]
7. Critical pressure (MPa): 4.42[7]
8. Octanol/water partition coefficient: 1.37[ 8]
9. Flash point (℃): 199.2[9]
10. Ignition temperature (℃): 577[10]
11. Solubility: Slightly soluble in cold water and benzene, soluble in hot water, hot benzene, ethanol, and ether. [11]
Toxicological data
1. Acute toxicity: rat oral LD50: 535mg/kg; mouse oral LD50: 308mg/kg; dog peritoneal cavity LDL0: 70mg/kg; guinea pig oral LD50: 450mg/kg; quail oral LD50: 562mg/ kg; 2. Other multiple dose toxicity: rat oral TDLo: 420mg/kg/28D-I; rat oral TDLo: 2940mg/kg/3W-I; 3. Mutagenicity: Mutant microbial test: bacteria-rat Salmonella typhi, 500μg/plate; DNA repair test: Bacillus subtilis, 5mg/disc;
4. Acute toxicity[12] LD50: 536mg/kg (rat oral administration)
5. Irritation No data available
6. Mutagenicity [13] Microbial mutagenicity: Salmonella typhimurium 10 μmol/dish. DNA repair: Bacillus subtilis 5mg/dish.
Ecological data
1. This substance may be harmful to the environment. It is recommended not to let it enter the environment.
2. Ecotoxicity[14] LC50: 96mg/L (48h) (medaka)
3. Biodegradability No data yet
4. Non-biodegradability[15] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 1.7d (theoretical).
Molecular structure data
1. Molar refractive index: 37.03
2. Molar volume (cm3/mol): 103.5
3. Isotonic specific volume (90.2K ): 288.5
4. Surface tension (dyne/cm)��60.3
5. Polarizability: 14.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 69.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 132
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product can cause blood poisoning that is stronger than aniline and cause cyanosis. Rat oral LD50900mg/kg. When the skin is contaminated by m-nitroaniline, if it is scrubbed with solvents such as ethanol, the absorption area and absorption speed will increase, causing danger. Therefore, polyethylene glycol should be used for scrubbing. Because it is highly toxic to the blood, special care must be taken when handling powders. The maximum allowable concentration in the air in the workplace is 1×10-6. Production equipment should be sealed to prevent running, emitting, dripping and leaking. Wear protective gear when operating. Flush with plenty of water for 15 minutes after contact. If it splashes into your eyes, please consult a doctor. Wash your contaminated clothes before wearing them again.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidants, acids, acid chlorides, acid anhydrides, chloroform, strong reducing agents
4. Conditions to avoid contact [18] Heating
5. Polymerization hazard[19] No polymerization
6. Decomposition products[20] Nitrogen oxides, ammonia
Storage method
1. Packed in iron drums lined with plastic bags, each drum is 50kg, and the drum should be labeled with “Violent Drugs”. The container should be sealed and placed in a cool, dry and ventilated place. Do not expose it to the sun, do not mix it with organic matter, flammable substances or food, and keep it away from fire and heat sources. Handle it with care when transporting. Store and transport according to the regulations for highly toxic substances.
2. Storage precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, reducing agents, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Using nitrobenzene as raw material, mixed acid with nitric acid and sulfuric acid is used for nitration to generate m-dinitrobenzene, which is refined with sulfurous acid to obtain m-dinitrobenzene finished product. Use sulfur and sodium sulfide as raw materials to prepare sodium polysulfide. Use sodium polysulfide to reduce m-dinitrobenzene into m-nitroaniline. The reaction product is recrystallized and filtered to obtain the finished product. Raw material consumption quota: nitrobenzene 1319kg/t, nitric acid 845kg/t, sulfuric acid 2050kg/t, sodium sulfide 707kg/t, sulfur 271kg/t.
![]()
![]()
Purpose
1. Used as dye intermediate and organic synthesis, and also used for color inspection of pine wood. Mainly used in organic synthesis as an intermediate for pesticides, dyes, medicines, color developers, plastics, synthetic fibers and auxiliaries.
2. Used as orange base R for ice dyeing and for preparing norophenol AS-BS. It is used to produce dyes such as Vermilion R, Lightfast Brilliant Yellow 10GX, Brown Salt V, Disperse Yellow SE-5R, Disperse Orange S-RFL, Disperse Scarlet SE-GFL, Disperse Scarlet S-BGL, Disperse Red S-ZGFL, etc.
3. Used as a dye intermediate and in organic synthesis, and also used to test the color of pine wood. [22]