Methanol Methanol
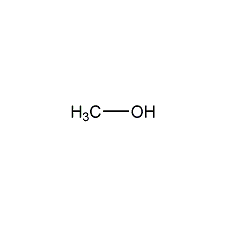

Structural formula
| Business number | 01ET |
|---|---|
| Molecular formula | CH4O |
| Molecular weight | 32.04 |
| label |
wood alcohol, Wood essence, methyl alcohol, Methyl alcohol, Carbinol, Wood alcohol, Wood spirit, Methyl hydroxide, Alcohol C1, Reagents for genetic engineering research, Dehydrating solvents for organic synthesis, car antifreeze, metal surface cleaner, alcohol denaturant, Aliphatic alcohols, ethers and their derivatives |
Numbering system
CAS number:67-56-1
MDL number:MFCD00004595
EINECS number:200-659-6
RTECS number:PC1400000
BRN number:1098229
PubChem number:24861543
Physical property data
1. Properties: colorless and transparent liquid with pungent odor. [1]
2. Melting point (℃): -97.8[2]
3. Boiling point (℃): 64.7[3]
4. Relative density (water = 1): 0.79[4]
5. Relative vapor Density (air=1): 1.1[5]
6. Saturated vapor pressure (kPa): 12.3 (20℃)[6]
7. Heat of combustion (kJ/mol): -723[7]
8. Critical temperature (℃): 240[8]
9. Critical pressure (MPa): 7.95[9]
10. Octanol/water partition coefficient: -0.82~- 0.77[10]
11. Flash point (℃): 12 (CC); 12.2 (OC) [11]
12. Ignition temperature (℃): 464[12]
13. Explosion upper limit (%): 36.5[13]
14. Lower explosion limit (%): 6[14]
15. Solubility: soluble in water, miscible with most alcohols, ether, etc. Organic solvents. [15]
16. Refractive index (n20ºC): 1.3284
17. Viscosity (mPa·s, 15ºC): 0.6405
18. Viscosity (mPa·s, 20ºC): 0.5945
19. Viscosity (mPa·s, 25ºC): 0.5525
20. Viscosity (mPa·s, 30ºC) : 0.5142
21. Flash point (ºC, open): 11
22. Flash point (ºC, closed): 12.0
23. Heat of evaporation ( KJ/mol, b.p.): 35.32
24. Heat of fusion (KJ/kg): 98.81
25. Heat of formation (KJ/mol, gas): -201.39
26. Heat of formation (KJ/mol, liquid): -238.82
27. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 2.51
28. Boiling point rising constant: 0.785
29. Electrical conductivity (S/m, 25ºC): 1.5×10-9
30. Thermal conductivity Rate (W/(m·K), 30ºC): 21.3527
31. Volume expansion coefficient (K-1, 20ºC): 0.00119
32 .Volume expansion coefficient (K-1, 55ºC): 0.00124
The 2OH group combines with hydrogen, and the formic acid generated during oxidation is further oxidized to CO2; ③ Methanol does not easily react with chlorine and bromine, but easily interacts with its aqueous solution, initially generating dichloromethyl ether (CH2Cl) 2O, which is converted into HCHO due to the action of water With HCl; ④ Heated with alkali and lime to produce hydrogen and sodium formate; CH3OH+NaOH→HCOONa+2H2; ⑤ Distilled with zinc powder, decomposed to produce CO and H2O.
2. Methanol is a toxic chemical product that has a significant anesthetic effect and is the most serious harm to the optic nerve. Drinking 5~10ml/kg of methanol can cause severe poisoning, more than 10ml/kg can cause blindness, and drinking 30ml/kg can cause death. Methanol can invade the human body through the digestive tract, respiratory tract and skin, causing poisoning. Inhaling concentrated methanol vapor often causes blurred vision and eye pain in addition to the unique symptoms of intoxication and headache. Some of these symptoms can occur within a few hours. In severe cases, dizziness, difficulty breathing, stomach pain, hernia, constipation, and sometimes bleeding may occur, and it may take several days to recover. During this period, it can also cause fatigue and discomfort, and in severe cases, cyanosis can occur. Whether acute or chronic, it takes a long time to recover. The maximum allowable concentration of methanol vapor in the air in the workplace is 260mg/m3. The protection method is to seal the production equipment and strictly prevent entry, eyes or wounds. If stuck, rinse quickly with water. People with acute poisoning should move to fresh air quickly. Severe cases should be sent to the hospital for treatment.
3. It is moderately toxic. Mainly acts on the nervous system and has anesthetic effect. It can be absorbed by the skin, drink or inhale the vapor and cause poisoning. It is characterized by irritating the optic nerve and retina, leading to eye blindness. Ethanol can be quickly decomposed and eliminated in the body, while methanol is excreted slowly, so it is cumulative. Inhaling methanol vapor can irritate the eyes, nose and throat, causing dizziness, headache, intoxication, tearing and blurred vision. In severe cases, anesthesia, difficulty breathing, nausea, vomiting, stomach pain, hernia, bladder pain, constipation, and sometimes bleeding may occur. Generally, drinking 5~10ml by mistake can cause severe poisoning, 15ml can cause blindness, and about 30ml can cause death. The oral lethal dose for rabbits is 10ml/kg. The olfactory threshold concentration is 140mg/m3. TJ36-79 stipulates that the maximum allowable concentration in workshop air is 50 mg/m3.
4. Stability[25] Stable
5. Incompatible substances[26] Acids, acid anhydrides, strong oxidants, alkali metals
6. Polymerization hazards [27] No polymerization p>
Storage method
Storage Precautions[28] Stored in a cool, well-ventilated dedicated warehouse, away from fire and heat sources. The storage temperature should not exceed 37°C and the container should be kept sealed. They should be stored separately from oxidants, acids, alkali metals, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Industrial synthesis of methanol almost all uses the method of pressurized catalytic hydrogenation of carbon monoxide. The process includes gas generation, synthetic purification, methanol synthesis and crude methanol distillation. The purification process of crude methanol includes distillation and chemical treatment. Chemical treatment mainly uses alkali to destroy impurities that are difficult to separate during distillation and adjust the pH value; distillation mainly removes volatile components such as dimethyl ether, as well as difficult-to-volatile components such as ethanol, high-carbon alcohols and water. The purity after crude distillation can generally reach more than 98%.
2. Reduce the moisture content of industrial methanol to less than 0.01% by distillation. Then treat it with sodium hypoiodite to remove the acetone. Pure methanol is obtained by distillation.
3. The preparation of BV-III grade methanol mainly adopts distillation process. Using industrial methanol as raw material, high-purity methanol products are obtained through distillation, ultra-clean filtration, and ultra-clean packaging.
4. Generally, industrial methanol is used as the raw material, and the water is removed by distillation under normal pressure. The temperature at the top of the tower is controlled to be 64-65°C, and the insoluble matter is removed by filtration.
Purpose
1. One of the basic organic raw materials. It is mainly used to manufacture a variety of organic products such as formaldehyde, acetic acid, methyl chloride, methylamine and dimethyl sulfate. It is also a raw material for pesticides (insecticides, acaricides), medicines (sulfonamides, synmycin, etc.), and one of the raw materials for the synthesis of dimethyl terephthalate, methyl methacrylate and methyl acrylate. It is also an important solvent and can also be mixed with gasoline and used as an alternative fuel. Since the 1980s, methanol has been used to produce products such as gasoline octane additive methyl tert-butyl ether, methanol gasoline, methanol fuel, and methanol protein, which has greatly promoted the development of methanol production and market demand.
2. Used as coatings, varnishes, shellac, inks, adhesives, dyes, alkaloids, cellulose acetate, cellulose nitrate, ethyl cellulose, polyvinyl butyral, etc. Solvent. It is also a raw material for manufacturing pesticides, medicines, plastics, synthetic fibers and organic chemical products such as formaldehyde, methylamine, methyl chloride, dimethyl sulfate, etc. Others are used as automobile antifreeze, metal surface cleaner and alcohol denaturant.
3. Methanol is a cleaning and degreasing agent. MOS grade is mainly used for discrete devices and medium and large-scale integrated circuits. BV-III grade is mainly used for very large-scale integrated circuit technology.
4. Used as analytical reagents, such as solvents, methylation reagents, and chromatographic analysis reagents. Also used in organic synthesis.
5. Used in the electronics industry, often used as cleaning and degreasing agents.
6. Methanol is usually a better solvent than ethanol and can dissolve many inorganic salts.
7. Mainly used for making formaldehyde, flavors, dyes, medicines, gunpowder, antifreeze, solvents, etc. [29]
Cleanse with degreaser.
6. Methanol is usually a better solvent than ethanol and can dissolve many inorganic salts.
7. Mainly used for making formaldehyde, flavors, dyes, medicines, gunpowder, antifreeze, solvents, etc. [29]