Methyl anthranilate
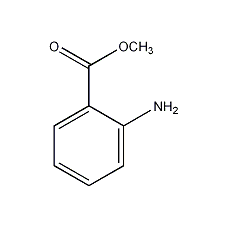

Structural formula
| Business number | 03PD |
|---|---|
| Molecular formula | C8H9NO2 |
| Molecular weight | 151.16 |
| label |
2-Aminobenzoic acid methyl ester, Methyl anthranilate, Methyl 2-aminobenzoate, 2-Aminobenzoic acid methyl ester, food additives, Flavor enhancer |
Numbering system
CAS number:134-20-3
MDL number:MFCD00007710
EINECS number:205-132-4
RTECS number:CB3325000
BRN number:606965
PubChem number:24854171
Physical property data
1. Physical property data
1. Properties: colorless to light yellow liquid with blue fluorescence, orange blossom aroma and slightly bitter spicy taste.
2. Density (g/mL, 25/4℃): 1.168
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 24-25
5. Boiling point (ºC, 0.67kpa or 5 mmHg): 256
6. Refractive index (n 20D): 1.581
7. Flash point (ºC): 104
8. Specific optical rotation Degree (ºC): Uncertain
9. Autoignition point or ignition temperature (ºC) is uncertain
10. Vapor pressure (kPa, 25ºC): Uncertain
11. Saturated vapor pressure (kPa, 60ºC): Uncertain
12. Heat of combustion (KJ/mol): Uncertain
13. Critical temperature (ºC): Uncertain
14. Critical pressure (KPa): Uncertain
15. Log value of oil-water (octanol/water) partition coefficient: Uncertain
16. Explosion upper limit (%, V/V): Uncertain
17. Explosion lower limit (%, V/V): Uncertain
18. Solubility: Soluble in ethanol , ether and other organic solvents, slightly soluble in water and glycerol.
Toxicological data
Skin/eye irritation data
Rabbit skin contact: 500mg/24H moderate reaction
Acute toxicity data:
Rat oral LD50: 2910mg /kg
Mouse oral LD50: 3900mg/kg
Rabbit skin LD50: >5mg/kg
Guinea pig oral LD50: 2780mg/kg
p>
Reproduction data:
8 days before mating of male mice or 21 days before mating of female mice TDLo: 34800mg/kg
Mutation data:
Bacteria-Bacillus subtilis DNA repair: 23mg/disc
Ecological data
None
Molecular structure data
Molecular property data:
1. Molar refractive index: 42.26
2. Molar refractive index: 42.26�� Volume (cm3/mol): 129.6
3. Isotonic specific volume (90.2K): 337.9
4. Surface tension (3.0 dyne/ cm): 46.2
5, Polarizability (0.5 10-24cm3): 16.75
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 4
6. Topological molecule polar surface area 52.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 147
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless to light yellow liquid. Melting point 24-25℃, boiling point 256℃, 135.5℃ (2.0kPa), relative density 1.1682 (18/4℃), refractive index 1.5820-1.5840. Soluble in ether, benzyl benzoate, diester phthalate, fixed oil, propylene glycol, volatile oil. Dissolve in 6 times the volume of 60% ethanol. Slightly soluble in water, insoluble in glycerol. It has blue fluorescence and will change color when exposed to light for a long time.
2. Found in burley tobacco leaves.
3. Naturally found in cocoa, wine, and strawberries.
Storage method
None
Synthesis method
1. In nature, methyl anthranilate is found in tower flower oil, neroli oil, ylang ylang, jasmine oil, tuberose oil, etc. Industrial production is generally obtained by esterification of anthranilic acid and methanol. Heat the methanol solution of anthranilic acid to 65°C, add sulfuric acid dropwise, and react at 75°C to generate sulfate of methyl anthranilate. Then neutralize with sodium hydroxide solution to precipitate methyl anthranilate. After extraction with toluene, washing the toluene extract and evaporating toluene, the crude methyl anthranilate is rectified under reduced pressure in the presence of sodium carbonate, and the finished fraction is cooled to below 12-15°C to precipitate. Methyl anthranilate. Another preparation method is to obtain this product from phthalic anhydride through ammoniation, degradation and esterification.
2.It is prepared by esterifying anthranilic acid and methanol under acidic conditions and neutralizing with alkali. Or it can be obtained by ammoniation, degradation and esterification of phthalic anhydride.
3. Tobacco: BU, 14.
Purpose
1. This product can be used as an intermediate for pesticides and saccharin. Methyl anthranilate has the sweet aroma of tower flowers, and has a grape-like aroma when diluted. It is used in the blending of cheap artificial flower oils, soap flavoring or grape essence. my country’s GB 2760-86 stipulates that edible spices are allowed to be used. Mainly used to prepare grape, citrus, loganberry, strawberry and watermelon flavors. Anthranilates can react with aldehydes to form valuable soap flavorings, such as hydroxyaromatic aldehydes and methyl anthranilate. The following spices are obtained. This spice is already commercially available in China under the trade name Aurantiol. The oral LD50 is 2910 mg/kg in rats and 3900 mg/kg in mice.
2.Used to prepare grape, cherry, strawberry, watermelon, citrus and other food flavors and wine flavors. The amount used in chewing gum is 2200mg/kg; in candies 56mg/kg; Pudding category 23mg/kg; Cold drinks 21mg/kg ; 20mg/kg in baked goods; 16mg/kg in soft drinks ;0.2mg/kg in alcoholic beverages.