N-(2-Hydroxyethyl)iminodiacetic acid N-(2-Hydroxyethyl)iminodiacetic Acid
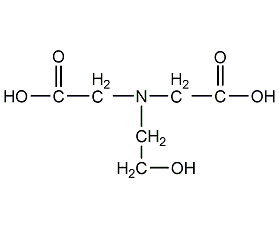

Structural formula
| Business number | 026M |
|---|---|
| Molecular formula | C6H11NO5 |
| Molecular weight | 177.16 |
| label |
(2-Hydroxyethyl)iminodiacetic acid, N-(carboxymethyl)-N-(2-hydroxyethyl)glycine, N-Hydroxyethylimodiacetic acid, 2-Hydroxyethylaminodiacetic acid, Ethanol diglycine, HOCH2CH2N(CH2COOH)2 |
Numbering system
CAS number:93-62-9
MDL number:MFCD00004293
EINECS number:202-263-9
RTECS number:AI1925000
BRN number:1780628
PubChem number:24878686
Physical property data
1. Characteristics: white granular crystals or crystalline powder
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC):168 ℃(180℃ decomposition)
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive index: undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºCOil and water (octanol/Log value of partition coefficient (water): undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility: Easily soluble in hot water, slightly soluble in cold water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index:38.23
2. Molar volume (m3/ mol):121.5
3. Isotonic specific volume (90.2K): 356.8
4. Surface tension (dyne/cm): 74.2
5. Polarizability(10-24cm3):15.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -3.5
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 98.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 153
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
Dissolve 60g chloroacetic acid in 200ml water, neutralize with 25g potassium carbonate, add 15g ethanolamine and 40g potassium carbonate, heat and reflux for 3 hours, cool and acidify to pH=2 with concentrated hydrochloric acid. After cooling, hydroxyethyl imine diacetic acid crystals precipitate. After recrystallization with water, 22g of finished product was obtained with a yield of 50%.
Purpose
As complexing agent.
t; FONT-FAMILY: 宋体; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial; mso-bidi-font-family: Arial”>): 121.5
3. Isotonic specific volume (90.2K): 356.8
4. Surface tension (dyne/cm): 74.2
5. Polarizability(10-24cm3):15.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -3.5
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 98.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 153
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
Dissolve 60g chloroacetic acid in 200ml water, neutralize with 25g potassium carbonate, add 15g ethanolamine and 40g potassium carbonate, heat and reflux for 3 hours, cool and acidify to pH=2 with concentrated hydrochloric acid. After cooling, hydroxyethyl imine diacetic acid crystals precipitate. After recrystallization with water, 22g of finished product was obtained with a yield of 50%.
Purpose
As complexing agent.