N,N-Dihydroxymethylurea
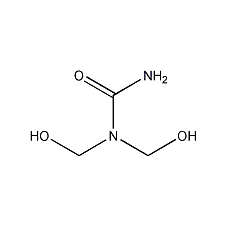

Structural formula
| Business number | 03TJ |
|---|---|
| Molecular formula | C3H8N2O3 |
| Molecular weight | 120.11 |
| label |
1,3-bis(hydroxymethyl)urea, 1,3-Bishydroxymethylurea, Hydroxymethylurea, 1,3-Bis(hydroxymethyl)urea, N,N’-Dimethylolurea, 1,3-Dimethylolurea, aliphatic compounds |
Numbering system
CAS number:140-95-4
MDL number:MFCD00014414
EINECS number:205-444-0
RTECS number:YS2716350
BRN number:1811870
PubChem number:24893902
Physical property data
1. Physical property data:
1.Characteristics: white powder
2.Flash point (℃): 100
3.Melting point (℃): 110-115
4.Solubility: Solubility in water 150 g/l, soluble in ethanol.
Toxicological data
None yet
Ecological data
3. Ecology Data:
1, other harmful effects: This substance may be harmful to the environment, special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1, Molar refractive index: 26.30
2, molar volume (cm3/mol): 81.1
3, isotonic specific volume (90.2K): 244.7
4, surface tension (dyne/cm): 82.8
5, polarizability (10-24cm3): 10.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -1.8
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 81.6
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 66.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Storage method
2-8Store at ℃.
Synthesis method
Can be prepared from urea and formaldehyde in the presence of salts or alkaline catalysts, and continue the reaction to obtain amino resin.
Purpose
It is one of the intermediates in the reaction of urea and formaldehyde to prepare amino resin. The hydroxymethyl group is reactive.
cm 0pt; TEXT-ALIGN: left; mso-pagination: widow-orphan” align=”left”>Preparation of amino resin from the reaction of urea and formaldehyde One of the intermediates, hydroxymethyl is reactive.