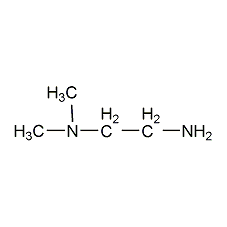
N,N-Dimethyltetradecylamine N,N-Dimethyltetradecylamine
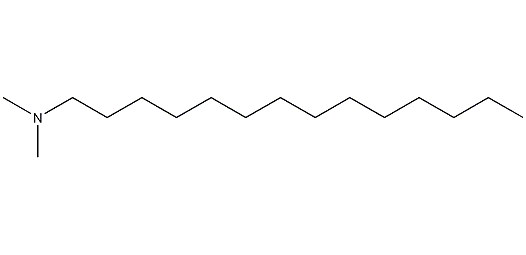

Structural formula
| Business number | 036K |
|---|---|
| Molecular formula | C16H35N |
| Molecular weight | None yet |
| label |
N,N-dimethyl-1-tetradecylamine, Dimethyltetradecylamine, Tetradecyldimethyltertiary amine, Dimethylmyristylamine, 1-(Dimethylamino)tetradecane, N,N-Dimethylmyristylamine, preservative, additive, fungicides, Extracting agent, Dispersant, flotation agent, linear compound |
Numbering system
CAS number:112-75-4
MDL number:MFCD00053736
EINECS number:204-002-4
RTECS number:None
BRN number:1748246
PubChem number:24865912
Physical property data
1. Character: Undetermined
2. Density (g/mL,20℃):0.795
3. Relative vapor density (g/mL,Air =1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC,normal pressure): Undetermined
6. Boiling point (ºC,2mmHg:148
7. Refractive index:1.441 ; mso-bidi-font-family: Arial; mso-font-kerning: 0pt”>
8. Flashpoint (ºC): 131
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of the partition coefficient for water: undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
None
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 79.89
2. Molar volume (m3/mol):299.8
3. isotonic specific volume (90.2K):694.3
4. Surface Tension (dyne/cm):28.7
5. Polarizability(10-24cm3):31.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 13
5. Number of tautomers: none
6. Topological molecule polar surface area 3.2
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 132
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
None
Purpose
Mainly used in preservatives, fuel additives, bactericides, rare metal extractants, pigment dispersants, mineral flotation agents, cosmetic raw materials, etc. .
94.3
4. Surface Tension (dyne/cm):28.7
5. Polarizability(10-24cm3):31.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 13
5. Number of tautomers: none
6. Topological molecule polar surface area 3.2
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 132
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
None
Purpose
Mainly used in preservatives, fuel additives, bactericides, rare metal extractants, pigment dispersants, mineral flotation agents, cosmetic raw materials, etc. .