N,N’-Diphenylurea N,N’-Diphenylurea


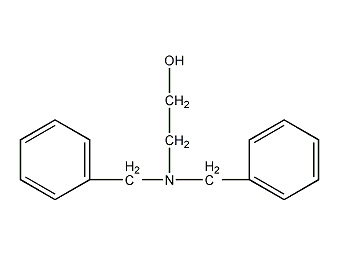
Structural formula
| Business number | 02LM |
|---|---|
| Molecular formula | C13H12N2O |
| Molecular weight | 212.25 |
| label |
1,3-Diphenylurea, diphenylurea, Diphenylurea, carbonanilide, N,N’-Diphenylurea, Carbanilide, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:102-07-8
MDL number:MFCD00003017
EINECS number:203-003-7
RTECS number:FD9800000
BRN number:782650
PubChem number:24848383
Physical property data
1. Properties: colorless prismatic crystals.
2. Density (g/mL, 25℃): 1.239
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 239-241
5. Boiling point (ºC, normal pressure): 262
6. Boiling point (ºC, kpa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 260
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V) : Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in glacial acetic acid, ether, slightly soluble in pyridine, very slightly Soluble in water, alcohol, acetone and chloroform.
Toxicological data
1. Acute toxicity: Rat oral LD50: 500mg/kg
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 65.07
2. Molar volume (cm3/mol): 169.8
3. Isotonic specific volume (90.2K ): 465.4
4. Surface tension (dyne/cm): 56.3
5. Dielectric constant:
6. Dipole moment��10-24cm3):
7. Polarizability: 25.79
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 41.1
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 196
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Originated from the condensation of aniline and urea.

Purpose
It is mainly used as an intermediate of dapsamide, and its chlorosulfonated product can be used as the main raw material of sulfamethoxazole.