o-Amino-p-cresol 2-Amino-4-methylphenol
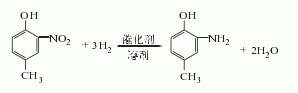
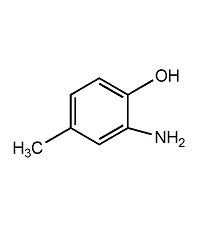
Structural formula
| Business number | 02A0 |
|---|---|
| Molecular formula | C7H9NO |
| Molecular weight | 123.15 |
| label |
2-amino-4-methylphenol; 3-methyl-6-hydroxyaniline, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:95-84-1
MDL number:MFCD00007699
EINECS number:202-457-3
RTECS number:SJ6078000
BRN number:606494
PubChem number:24848690
Physical property data
1. Properties: white crystals, industrial products are gray-white crystals.
2. Density (g/mL, 20℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 135~137
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in organic solvents such as ethanol, ether, and chloroform . Slightly soluble in water and benzene. Easily soluble in hot water.
Toxicological data
1. Mutagenicity Microorganism Salmonella typhimurium mutation: 333μg/plate;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 37.19
2. Molar volume (cm3/mol): 106.4
3. Isotonic specific volume (90.2K ): 285.7
4. Surface tension (dyne/cm): 51.9
5. Polarizability (10-24cm3): 14.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 11
6. Topological molecule polar surface area 46.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 94.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocentersNumber of � bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants and acids.
Toxic. Irritating to skin. The equipment in the production site must be sealed and well ventilated. Production operators should wear protective equipment.
Easily oxidized and discolored when exposed to air. Irritating.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants and acidic substances, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Packed in iron drums or cardboard drums lined with plastic bags. Net weight per barrel is 25kg or 50kg. Store and transport according to general chemical regulations.
Synthesis method
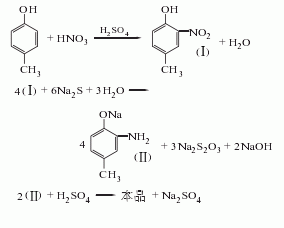
O-nitro-p-cresol is obtained by reduction with alkali sulfide or catalytic hydrogenation. Starting from the nitration of p-cresol, the raw material consumption quota is: p-cresol industrial product 963kg/t, nitric acid (96%) 661kg/t, sulfuric acid (92.5%) 2127kg/t, alkali sulfide (60%) 2425kg/t, soda ash 20kg/t t.
1. Use p-cresol as raw material, prepare a certain concentration of mixed acid with nitric acid and sulfuric acid for nitrification. After the reaction is completed, the waste acid is separated, washed and distilled and reduced with alkali sulfide, and finally the finished product is obtained by acid precipitation, crystallization and drying.
2. Hydrogenation reduction method is the same as method 1, but is carried out in the presence of a catalyst. Hydrogenation reduction is used instead of alkali sulfide reduction.
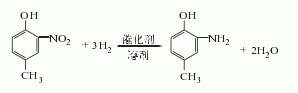
Purpose
Dye intermediates. Used to prepare polyester fiber fluorescent whitening agent DT and plastic fluorescent whitening agent PF.