o-Trifluoromethylbenzoic acid
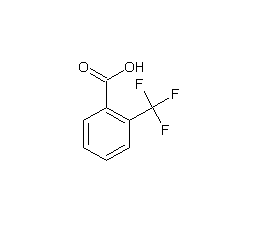

Structural formula
| Business number | 04TD |
|---|---|
| Molecular formula | F6NaSb |
| Molecular weight | 190 |
| label |
None yet |
Numbering system
CAS number:433-97-6
MDL number:MFCD00002476
EINECS number:240-989-8
RTECS number:None
BRN number:976984
PubChem number:24851804
Physical property data
Toxicological data
Not available
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 38.16
2. Molar volume (cm3/mol): 135.4
3. Isotonic specific volume (90.2K ): 326.6
4. Surface tension (dyne/cm): 33.7
5. Polarizability (10-24cm3): 15.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 200
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Irritating substances. Irritating to eyes, respiratory tract and skin. In case of contact with eyes, rinse immediately with plenty of water and seek medical attention. Use suitable gloves and protective glasses or face shield.
Storage method
None yet
Synthesis method
1. Ethyl o-carboxybenzoate method: Ethyl o-carboxybenzoate is used as raw material and reacts with sulfur tetrafluoride to obtain o-trifluoromethylbenzoyl chloride. The yield is 80%~83%; o-trifluoromethylbenzoic acid is obtained through hydrolysis.
2. O-Trifluoromethylfluorobenzene method: Using o-trifluoromethylchlorobenzene as raw material, it is obtained by Grignard reaction, with a yield of 90%.
Purpose
Used in the synthesis of fluorine-containing fine chemicals such as medicines and pesticides.