p-chlorobenzophenone
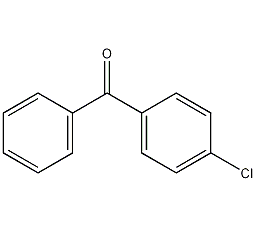

Structural formula
| Business number | 03PR |
|---|---|
| Molecular formula | C13H9ClO |
| Molecular weight | 216.67 |
| label |
4-Chlorobenzophenone, 4-Chlorodiphenylketone, (4-chlorophenyl)phenyl-methanon, photoinitiator, catalyst, photoinitiator, Environmental catalyst |
Numbering system
CAS number:134-85-0
MDL number:MFCD00000622
EINECS number:205-160-7
RTECS number:None
BRN number:512043
PubChem number:24892495
Physical property data
None
Toxicological data
None
Ecological data
None
Molecular structure data
Molecular property data: 1、 Molar refractive index60.94 2、 Molar volume (m3/mol): 179.4 3、 Isotonic specific volume(90.2K):462.7 4、 Surface tension(3.0 dyne/cm):44.1 5、 Polarizability0.5 10-24 cm3):24.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 213
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
White crystal powder. Melting point 75℃℃
Storage method
None
Synthesis method
Obtained from the condensation of benzoyl chloride and chlorobenzene: In a dry reaction pot, add chlorobenzene and anhydrous aluminum trichloride, stir and raise the temperature to 50°C, and add benzoyl chloride dropwise. After the addition, react at 100-110°C for 5.5 hours. Slowly add slightly acidic ice water, stir well and let it stand. Remove the supernatant, filter, wash until neutral, and spin dry to obtain 4-chlorobis. benzophenone.
Purpose
Pharmaceutical intermediates
urcevalue=”24″ hasspace=”False” negative=”True” numbertype=”1″ tcsc=”0″ w:st=”on”>-24cm3):24.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 213
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
White crystal powder. Melting point 75℃℃
Storage method
None
Synthesis method
Obtained from the condensation of benzoyl chloride and chlorobenzene: In a dry reaction pot, add chlorobenzene and anhydrous aluminum trichloride, stir and raise the temperature to 50°C, and add benzoyl chloride dropwise. After the addition, react at 100-110°C for 5.5 hours. Slowly add slightly acidic ice water, stir well and let it stand. Remove the supernatant, filter, wash until neutral, and spin dry to obtain 4-chlorobis. benzophenone.
Purpose
Pharmaceutical intermediates