Pentaerythritol
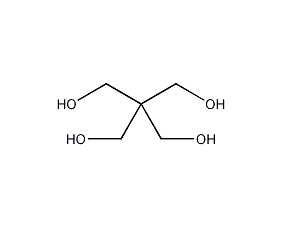
Structural formula
| Business number | 0386 |
|---|---|
| Molecular formula | C5H12O4 |
| Molecular weight | 136.15 |
| label |
Tetrahydroxymethylmethane, Tetrahydroxymethylalkane, Monopentaerythritol, 2,2-Bis(hydroxymethyl)-1,3-propanediol, C(CH2OH)4, gas chromatography stationary solution, alcohol solvents, Heat stabilizers, Plasticizer |
Numbering system
CAS number:115-77-5
MDL number:MFCD00004692
EINECS number:204-104-9
RTECS number:RZ2490000
BRN number:1679274
PubChem number:24898496
Physical property data
1. Properties: White powdery crystal, flammable, easily esterified by general organic acids, no reaction when boiled with dilute caustic soda solution.
2. Density (g/mL): 1.399
3. Melting point (ºC): 262
4. Boiling point (ºC, 4.0KPa): 276
5. Refractive index (20ºC): 1.54~1.56
6. Flash point (ºC): 102
7. Fire point (ºC): about 370
8. Autoignition point or ignition temperature (ºC): 450 (powder)
9. Saturated vapor pressure (kPa, 276ºC): 4.0
10 . Heat of combustion (KJ/kg): 2765
11. Heat of generation (KJ/kg): 948
12. Heat of sublimation (KJ/kg): 131.5
13. Heat of fusion (KJ/kg): about 21
14. Heat of evaporation (KJ/kg): about 92
15. Lower explosion limit (%, V/ V): 30 (g/m3)
16. Solubility: soluble in water, slightly soluble in ethanol, insoluble in benzene, carbon tetrachloride, ether, petroleum ether, etc.
17. Relative density (20℃, 4℃): 1.39730
18. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2905.8
19. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -776.7
20. Crystalline phase standard combustion heat (enthalpy) (kJ·mol-1): -2761.9
21. Crystalline phase standard claim heat (enthalpy) (kJ·mol -1):-920.6
Toxicological data
Toxicity Classification Poisoning Acute Toxicity Oral – Rat LD50: 12600 mg/kg; Oral – Mouse LD50: 4097 mg/kg. Irritation Data Skin – Rabbit 500 mg/24 hours Mild; Eyes – Rabbit 126 mg Mild. Large doses of oral administration can cause diarrhea, but no skin irritation or inflammation has been reported. The dust is also harmless to humans.
Ecological data
Slightly harmful to water.
Molecular structure�Data
1. Molar refractive index: 37.51
2. Molar volume (cm3/mol): 139.1
3. Isotonic specific volume (90.2K ): 339.2
4. Surface tension (dyne/cm): 35.3
5. Polarizability (10-24cm3 ): 14.87
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -2.4
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 80.9
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 51.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It does not decompose under normal temperature and pressure. Avoid contact with strong oxidants, strong acids, acid chlorides and acid anhydrides. Flammable, the hydroxyl group in pentaerythritol can undergo esterification, nitration, halogenation, etherification and oxidation reactions. Form complexes with metals. 2. Basically non-toxic. It does not produce metabolic changes in the human body, but high blood sugar or diarrhea may occur when taking high doses. No irritation or inflammation occurs in contact with skin and eyes with saturated solutions of pentaerythritol.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. 2. Packed in polypropylene woven bags or sacks covered with plastic bags, 25kg per bag. When the concentration of pentaerythritol dust in the air reaches more than 30g/m3, it can form an explosive mixture with air, and explode when it exceeds 400°C. Therefore, it should be stored in a cool, dry and ventilated place, away from moisture and fire. Store and transport according to general chemical regulations.
Synthesis method
1. It is obtained by reacting formaldehyde and acetaldehyde as raw materials in the presence of an alkaline condensing agent. When sodium hydroxide is used as the condensing agent, it is called the sodium method. The molar ratio of raw materials is acetaldehyde: formaldehyde: alkali = 1.5: 6: 1.1-1.3. Add the sodium hydroxide solution to the 37% formaldehyde solution, add acetaldehyde under stirring, and react at 25-32°C for 6-7 hours. After neutralization and filtration, pentaerythritol is obtained. Raw material consumption quota: formaldehyde (37%) 2880kg/t, acetaldehyde 350kg/t.
![]()
When calcium hydroxide is used as the shrinkage mixture, it is called the calcium method. The molar ratio of raw materials is acetaldehyde: formaldehyde: lime = 1: 4.7: 0.7-0.8. Add formaldehyde solution, 20% acetaldehyde solution and 25% milk of lime into the reaction pot and react below 60°C. The color of the condensation liquid will change from gray to blue. Gradually lower the temperature to 45°C and put it into the acidification pot. Use 60-70% sulfuric acid to acidify the condensation liquid to a pH of 2-2.5, and then use a filter press to remove calcium sulfate. The filtrate is passed through an ion effect exchange column to remove residual calcium ions, and is concentrated under reduced pressure. The gas phase temperature is maintained below 70°C, and the vacuum degree is maintained at 77.3kPa. When crystallization begins to occur, put the concentrated liquid into a crystallization tank, stir to cool the crystallization, centrifuge, wash with water until the pH is 3, and dry the wet product with air flow to obtain the finished product. The calcium method has high consumption and many waste problems. Raw material consumption quota: formaldehyde (36.5%) 4700kg/t, acetaldehyde 550kg/t.
2.Pentaerythritol is produced from formaldehyde and acetaldehyde through a condensation reaction in the presence of an alkali catalyst:
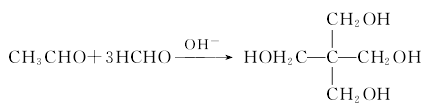
3. Preparation method:
![]()
In a reaction flask equipped with a stirrer, thermometer, , add 800g of paraformaldehyde (approximately equivalent to 26.7mol of formaldehyde), 5.5L of water, an acetaldehyde aqueous solution containing 210g of acetaldehyde (4.77mol), and add 180g of finely ground quicklime (3.22mol) in batches while stirring. Control the addition speed of quicklime so that the temperature of the reaction solution rises to 50°C in about 0.5h. Continue to heat quicklime until the maximum temperature does not exceed 55°C. After the addition was completed, the reaction was stirred for 3 hours. The mixture is light yellow in color. Filter, adjust the filtrate to dilute hydrochloric acid until it is acidic to litmus paper, decolorize with activated carbon and filter to obtain an almost colorless solution. Concentrate under reduced pressure until crystallization begins. Filter while hot and wash the filter paper with a small amount of hot water. Place in the refrigerator to crystallize. Filter to get the first batch of solids. The mother liquor continues to be concentrated and treated as above to obtain the second batch of products. Concentrate again to obtain the third batch of products. A total of about 400g of crude pentaerythritol product was obtained. Weigh with hot water containing a small amount of hydrochloric acid, decolorize with activated carbon, and concentrate appropriately. Follow the above method to precipitate crystals several times to obtain a total of 360 to 370 g of pentaerythritol (1), mp 257 to 260°C. [1]
Purpose
1. Used in the manufacture of pentaerythritol tetranitrate explosives, alkyd resins, and also used as heat stabilizers, plasticizers, etc. It is widely used in the coating industry to produce alkyd resins, synthetic high-grade lubricants, plasticizers, surfactants, and raw materials such as medicines and explosives.
2.Is an important polyol compound. It is widely used in the production of alkyd resins, flame retardant coatings, polyurethanes, and drying oils; it is also a synthetic plasticizer, flame retardant, surfactant, emulsifier, pesticide, antioxidant, high-grade lubricant, and pentaerythritol ester. , pentaerythritol polyether, polyester, PENT explosives and chlorinated polyether are the main raw materials.
3. Used as a raw material for the production of rosin ester in varnishes, paints and inks, and can be used as flame retardants, drying oils and aviation lubricants.
Pharmaceuticals, explosives and other raw materials.
2.Is an important polyol compound. It is widely used in the production of alkyd resins, flame retardant coatings, polyurethanes, and drying oils; it is also a synthetic plasticizer, flame retardant, surfactant, emulsifier, pesticide, antioxidant, high-grade lubricant, and pentaerythritol ester. , pentaerythritol polyether, polyester, PENT explosives and chlorinated polyether are the main raw materials.
3. Used as a raw material for the production of rosin ester in varnishes, paints and inks, and can be used as flame retardants, drying oils and aviation lubricants.