Piperazine hexahydrate

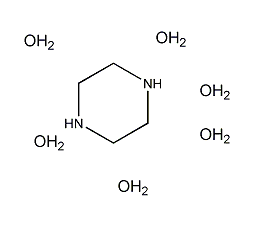
Structural formula
| Business number | 03V7 |
|---|---|
| Molecular formula | C4H10N2·6H2O |
| Molecular weight | 194.23 |
| label |
Diethylenediamine hexahydrate, Piperazine hexahydrate, Piperazine hexahydrate, Diethylenediamine hexahydrate, Heterocyclic compounds |
Numbering system
CAS number:142-63-2
MDL number:MFCD00149389
EINECS number:203-808-3
RTECS number:TM0850000
BRN number:4455527
PubChem ID:None
Physical property data
1. Physical property data
1. Appearance: white crystal
2. Flash point (ºC): 190°F
3. Melting point (ºC) ): 44-45 ºC
4. Boiling point (ºC, normal pressure): 145-156ºC
Toxicological data
2. Toxicological data:
1. Acute toxicity: Rat oral LDLo: 500 mg/kg;
Mouse oral LD50: 11200 mg/kg;
Mouse abdominal LD50: 300 mg/kg.
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 8
3. Number of hydrogen bond acceptors: 8
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 30.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 26.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 7
Properties and stability
Stable under normal temperature and pressure.
Low toxicity, oral administration of LD501900mg/kg to rats and oral administration of LD5011200mg/kg to mice. The maximum allowable concentration in the air is 5 mg/m3 (adipate).
Storage method
Seal tightly and store in a cool place away from light.
The inner liner is lined with plastic bags, and the outer cover is packed in a plastic barrel. Pay attention to sealing to prevent moisture absorption. During storage and transportation, it should be protected from rain, sun and fire. Store and transport according to general chemical regulations.
Synthesis method
Synthesize piperazine hydrochloride from chloroethanol through ammoniation and cyclization.�Then neutralize with sodium hydroxide to prepare piperazine hexahydrate, the reaction formula is as follows:

Purpose
This product is mainly used in the production of anthelmintic drugs piperazine phosphate, piperazine citrate, as well as fluphenazine, analgesia, and rifampicin. Piperazine hexahydrate is dissolved in alcohol. Add glacial acetic acid, stir and cool to precipitate piperazine acetate, which can be used to synthesize the hormone drug prednisone sodium phosphate. Piperazine hexahydrate can also be used to produce acetylpiperazine pharmaceutical intermediates. Piperazine is also used in the production of surfactant products such as wetting agents, emulsifiers, and dispersants, as well as antioxidants, preservatives, stabilizers, plastic processing aids, and rubber additives.