Potassium silver cyanide
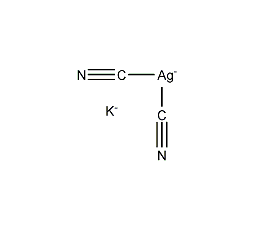

Structural formula
| Business number | 059K |
|---|---|
| Molecular formula | KAg(CN)2 |
| Molecular weight | 199.01 |
| label |
Potassium silver(I) cyanide, K[Ag(CN)2], fungicides, preservative |
Numbering system
CAS number:506-61-6
MDL number:MFCD00036297
EINECS number:208-047-0
RTECS number:TT5775000
BRN number:None
PubChem number:24863622
Physical property data
1. Properties: White crystal, sensitive to light.
2. Density (g/cm3, 25/4℃): 2.36
3. Relative steam density (g/cm3 , air = 1): not determined
4. Melting point (ºC): not determined
5. Boiling point (ºC, normal pressure): not determined
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºF): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC ): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined Determined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
1. Skin/eye irritation data: Standard Draize test rabbit direct contact with skin: 500mg/24HREACTION SEVERITY: severe;
Standard Draize test rabbit direct contact with eyes: 250ug/24HREACTION SEVERITY: severe;
p>
2. Acute toxicity: Rat oral LD50: 20900ug/kg, no detailed description except lethal dose;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 47.6
7.Number of heavy atoms: 6
8, Surface charge: 0
9, Complexity: 10
10, Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain the number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. The number of covalent bonding units: 4
Properties and stability
Use and store according to specifications. It will not decompose and avoid contact with oxides. Soluble in water, AgCN precipitates in acidic solutions.
Storage method
Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Dissolve pure silver with nitric acid, remove impurities, filter, add KCN or NaCN for precipitation, wash, add KCN for complexation, and finally filter, crystallize, centrifuge, and dry to obtain the finished product.
The solution containing KCN and AgCN was evaporated at room temperature.
Purpose
The coating has good weldability, high coating surface finish, and strong bonding with the base material. Can be used for surface silver plating of electronic devices. Also used as a fungicide and preservative.