Pyridine nitrogen oxide
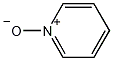
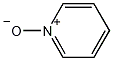
Structural formula
| Business number | 07A9 |
|---|---|
| Molecular formula | C5H5NO |
| Molecular weight | 95.10 |
| label |
Pyridine-N-oxide, Pyridine N-oxide, Pyridine oxynitride, pyridine oxide, 1-Pyridine oxide, Antibiotic synthesis raw materials |
Numbering system
CAS number:694-59-7
MDL number:MFCD00006194
EINECS number:211-774-6
RTECS number:UT6410000
BRN number:105257
PubChem number:24888050
Physical property data
1. Properties: crystalline.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 66
5. Boiling point (ºC, normal pressure): 270
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 143
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure ( kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in water.
Toxicological data
1. Acute toxicity: Mouse (peritoneal 0LD50: 1,425 mg/kg) Mouse (intravenous) LD50: 180 mg/kg Quail (oral 0LD50: 1 mg/kg Wild birds (oral 0LD50: 1 mg/kg
Due to salt The LD50 is 3,000 mg/kg, and the acute toxicity of BPA is the same as that of table salt.
Ecological data
Do not allow undiluted or large quantities of products that are slightly hazardous to water to come into contact with groundwater, waterways or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 25.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 50
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Keep away from oxides and water sources.
2.Hygroscopic. It emits extremely toxic fumes when heated to decompose.
Storage method
1. Store in a sealed container and place in a cool, dry place. Store away from oxidizing agents. Avoid contact with moisture and water.
Synthesis method
1. Prepared by oxidizing pyridine with peroxyphthalic acid.
2. In a 1-liter three-necked flask equipped with a stirrer, thermometer and dropping funnel, put 110 grams (1.39 mol) of pyridine, while stirring and maintaining 85°C, add 250 ml (285 g, 1.50 mol) of 40% peracetic acid (10% or 20% peracetic acid can also be used). It takes 50 to 60 minutes to complete. Continue stirring until the temperature of the mixture drops to 40°C. Use potassium iodide solution to check that the acetic acid does not contain peroxide, evaporate the acetic acid solution on a water vapor bath and under reduced pressure with a water pump. The residue (180-190 g) was distilled under a pressure of >1 mm, and the solid distillate was collected. The vacuum pump must be protected by a dry ice trap (about 60 ml of acetic acid produced by the dissociation of pyridine oxide acetate under low pressure may be collected in the cold trap). Use an oil bath to heat and do not let the temperature rise above 130°C. 103-110 g (78-83%) colorless solid was obtained.
Purpose
1. Pyridine N-oxide is an important organic synthesis intermediate and is often used in the synthesis of antibiotics and other drugs in the pharmaceutical industry, such as the synthesis of cefpirin.