Quinalizarin Quinalizarin
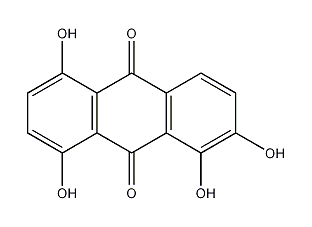

Structural formula
| Business number | 01SA |
|---|---|
| Molecular formula | C14H8O6 |
| Molecular weight | 272.21 |
| label |
1,2,5,8-Tetrahydroxyanthraquinone |
Numbering system
CAS number:81-61-8
MDL number:None
EINECS number:201-366-6
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Character: red needle crystal
2. Density (g/mL,25/4℃): Unsure
3. relativeVapor density (g/mL,Air=1): Unsure
4. Melting point (ºC):> 275
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Saturated vapor pressure (kPa,60ºC): Unsure
<P class=MsoNormal style="MARGIN: 0cm 0cm 0pt 36pt; TEXT-INDENT: -18pt; TEXT-ALIGN: left; mso-list: l0 level1 lfo1; tab-stops: list 36.0pt; mso-pagination: widow- orphan; mso-margi
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:It is reddish purple when dissolved in alkaline aqueous solution, yellow in acetic acid, blue-violet in sulfuric acid, and produces a matte red precipitate when diluted. Soluble in ethanol, very slightly soluble in various organic solvents, insoluble in water
Toxicological data
1, acute toxicity
Rat caliberLD:>500 mg/kg;
2, teratogenicity
Salmonella: 50ug/plate
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 66.19
2. Molar volume (m3/mol):152.7
3. isotonic specific volume (90.2K):495.7
4. Surface Tension (dyne/cm):110.8
5. Polarizability(10-24cm3):26.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.5
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 227
6. Topological molecular polar surface area 115
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 435
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored in a dry and dark place.
Synthesis method
None yet
Purpose
Determination of trace amounts of beryllium, magnesium, calcium, aluminum, lanthanum, thorium, cerium, praseodymium, neodymium, zirconium and boron. Mordant dyes for cotton.
el2 lfo1; tab-stops: list 36.0pt; mso-margin-top-alt: auto; mso-margin-bottom-alt: auto” align=left>2. Molar volume (m3/mol ): 152.7
3. isotonic specific volume (90.2K):495.7
4. Surface Tension (dyne/cm):110.8
5. Polarizability(10-24cm3):26.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.5
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 227
6. Topological molecular polar surface area 115
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 435
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored in a dry and dark place.
Synthesis method
None yet
Purpose
Determination of trace amounts of beryllium, magnesium, calcium, aluminum, lanthanum, thorium, cerium, praseodymium, neodymium, zirconium and boron. Mordant dyes for cotton.
Determination of trace amounts of beryllium, magnesium, calcium, aluminum, lanthanum, thorium, cerium, praseodymium, neodymium, zirconium and boron. Mordant dyes for cotton.