Sodium tetraphenylborate

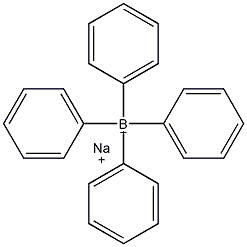
Structural formula
| Business number | 03W7 |
|---|---|
| Molecular formula | C24H20BNa |
| Molecular weight | 342.22 |
| label |
Sodium tetraphenylborate, Sodium tetraphenylborate, Sodium tetraphenylborate, Sodium tetraphenylboride, Tetraphenylboron sodium, Sodium tetraphenylboron, Na-TPB, Analytical identification reagents |
Numbering system
CAS number:143-66-8
MDL number:MFCD00011494
EINECS number:205-605-5
RTECS number:ED3362500
BRN number:3599783
PubChem number:24886345
Physical property data
1. Physical property data:
1. Character: white crystal. Odorless. Sensitive to light. Slightly hygroscopic.
2. Melting point (ºC): greater than 300ºC
3. Solubility: Soluble in water, acetone, acetonitrile, dihydrogen For methylformamide, the lower the temperature, the greater the solubility. Insoluble in benzene and tetrachloridecarbon. Precipitates with potassium, rubidium, cesium, silver, and mercury salts. Forms insoluble precipitate with organic bases.
Toxicological data
II. Toxicological data:
1. Acute toxicity: Rat oral LD50: 288 mg/kg Irritating
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 26
8. Surface charge: 0
9. Complexity: 308
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials: strong oxidizing agents
Storage method
Stored in a cool and dry place
Synthesis method
1. Use magnesium chips, brominated benzene, ether solution and iodine tablets to synthesize Grignard reagent. Its content is generally about 39%-43%. Then, react the Grignard reagent with trimethyl borate, hydrolyze, extract with ether, and filter. The filtrate was extracted with chloroform, salt was added to the extract, and a white precipitate was obtained by salting out (���Crude sodium phenylboronate). Then extract with acetone, filter out the salt, concentrate the filtrate under reduced pressure at room temperature, crystallize, recrystallize with acetone, and dry at 30-40°C to obtain the finished product.
2.Dissolve metal magnesium and bromobenzene in anhydrous ether to prepare a phenylmagnesium bromide solution. Add sodium tetrafluoroborate and diethyl ether to form a solution. The reaction mixture was placed on a water bath under vacuum and boiled. Add activated carbon to decolorize, filter, add sodium chloride, and precipitate sodium tetraphenylborate.
3.Fill the reaction tube with magnesium chips, then immerse it in refined anhydrous ether, and add iodine as an initiator. Slowly heat until the ether refluxes, and then slowly add the ether solution of bromobenzene slowly dropwise. Control the dropping speed to keep the liquid level in the tube boiling. When phenylmagnesium bromide is generated, the material can be discharged while dropping. Magnesium should be replenished in time when half of the consumption is reached. After the generated phenyl magnesium bromide is diluted with anhydrous ethyl ether, slowly add trimethyl borate anhydrous ether solution while stirring, and control the temperature at about 34°C. After the reaction is completed, let it stand, and separate the upper layer of ether and the lower solution. Below 10°C, add a small amount in portions to the sodium carbonate aqueous solution. After the addition is completed, continue stirring for half an hour. The process reaction formula is:

After standing, separate the ether layer , and then extract with diethyl ether until the pH value is 8 to 9. Combine the extracts, add activated carbon, heat and stir, filter while hot, distill under reduced pressure, steam out diethyl ether, methanol, and water in sequence, cool, and filter. Add water and activated carbon to the filtrate again to decolorize, distill under reduced pressure until the distillate is clear, cool, filter and decolorize, the ph value of the filtrate is not less than 8. Prepare a solution of crude sodium tetraphenylborate, add activated carbon, stir for 4 hours, filter and decolorize, heat and concentrate the filtrate until a small amount of salt precipitates, maintain the temperature at 90°C, add 90°C saturated brine with pH = 8 to 9 while stirring for salting. Analyze, filter while hot, and dry the crystals at 60°C. The obtained sodium tetraphenylborate was added in small amounts to acetone below 5°C in batches. After treatment with activated carbon, the acetone was recovered under reduced pressure until the residual liquid was turbid. Then it was reduced to dryness under normal temperature, filtered, washed with anhydrous ether, and dried. for the finished product.
Purpose
1. Preparation of carbonate polycondensation catalyst by transesterification method. It is also used for the determination of potassium, sodium and chlorine-containing organic compounds, such as the analysis of potassium in fertilizers and blood.
2. Used for the determination of potassium, sodium and several nitrogen-containing organic compounds.
3.Analytical reagent, use For the determination of potassium, ammonium, rubidium, cesium, mercury, thallium and nitrogen-containing organic compounds.