tert-Butylbenzene
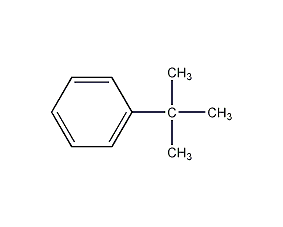
Structural formula
| Business number | 02D7 |
|---|---|
| Molecular formula | C10H14 |
| Molecular weight | 134 |
| label |
terbutylbenzene, phenylisobutane, Pseudobutylbenzene, Trimethylphenylmethane, aromatic compounds |
Numbering system
CAS number:98-06-6
MDL number:MFCD00008816
EINECS number:202-632-4
RTECS number:CY9120000
BRN number:1421537
PubChem number:24851757
Physical property data
1. Properties: colorless and transparent liquid.
2. Boiling point (ºC, 101.3kPa): 169.119
3. Boiling point (ºC, 2.93kPa): 70~75
4. Melting point (ºC ): -57.80
5. Relative density (g/mL, 20/4ºC): 0.8665
6. Relative density (g/mL, 25/4ºC): 0.86240
p>
7. Refractive index (n20D): 1.4927
8. Refractive index (25ºC ): 1.49024
9. Viscosity (mPa·s, 20ºC): 28.13
10. Viscosity (mPa·s, 30ºC): 27.14
11. Flash point (ºC): 34
12. Fire point (ºC): 450
13. Heat of vaporization (KJ/mol, 25ºC): -49.11
14. Heat of evaporation (KJ/mol, b.p.): -37.634
15. Heat of fusion (KJ/mol): -8.399
16. Heat of formation (KJ/mol, 25ºC ): 70.84
17. Heat of combustion (KJ/mol): 5874.9
18. Specific heat capacity (KJ/(kg·K), 21.27ºC): 1.78
19. Critical temperature (ºC): 374.1
20. Critical pressure (MPa): 2.979
21. Solubility (%, water, 20ºC): 0.0292
22. Vapor pressure (kPa, 25ºC): 0.28
23. Vapor pressure (kPa, 73.07ºC): 4.00
24. Vapor pressure (kPa, 102.449ºC) :13.33
25. Explosion lower limit (%,V/V): 0.7
26. Explosion upper limit (%,V/V): 5.7
27 . Solubility: Insoluble in water, miscible with benzene and acetone, easily soluble in organic solvents such as ethanol and ether.
28. Critical density (g·cm-3): 0.285
29. Critical volume (cm3·mol -1): 472
30. Critical compression factor: 0.261
31. Eccentricity factor: 0.266
32. Solubility Parameter (J·cm-3)0.5: 17.228
33. van der Waals area (cm2·mol -1): 1.166×1010
34. van der Waals volume (cm3·mol-1): 90.180
35. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5912.66
36. Gas phase standard claimed heat (Enthalpy) (kJ·mol-1): -23.26
37. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1 ): -5865.21
38. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -70.71
39. Liquid phase standard Entropy (J·mol-1·K-1): 278.7
40. Liquid phase standard free energy of formation (kJ·mol -1): 135.8
41. Liquid phase standard hot melt (J·mol-1·K-1): 240
Toxicological data
Acute toxicity: Rat oral LDL0: 10mL/kg; irritating to the skin, and its vapor is harmful if inhaled.
Ecological data
This substance may be harmful to the environment and it is recommended not to let it enter the environment.
Molecular structure data
1. Molar refractive index: 44.63
2. Molar volume (cm3/mol): 156.1
3. Isotonic specific volume (90.2K ): 355.3
4. Surface tension (dyne/cm): 26.8
5. Dielectric constant: 2.34
6. Dipole moment (10-24cm3):
7. Polarizability: 17.69
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 4.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 91.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants. Flammable, danger of burning on contact with oxidizing agents. Irritating to skin, avoid contact with eyes and skin.
2.This product is slightly toxic. Rat oral LD505000mg/kg.
3. Exist in smoke.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
This product is flammable and may burn when exposed to high heat, open flame, or contact with oxidants. See cumene for storage and transportation.
Synthesis method
1. The tert-butanol method is obtained by the hydrocarbonization of tert-butanol and benzene in the presence of aluminum trichloride. First mix benzene and aluminum trichloride, stir and cool to 5-6°C, slowly add the benzene solution of tert-butanol, keep the temperature at 8-11°C, and continue stirring for 4 hours after the addition. After the reaction is completed, destroy the aluminum trichloride with water and add benzene to extract. Pour the benzene extract into ice water, wash with water until neutral, steam out benzene and low boiling matter, and then distill to collect the 165-174°C fraction, which is tert-butylbenzene. The yield is over 60%. Benzene and chlorotert-butane were reacted in the presence of aluminum trichloride at 0-5°C for 1 hour, and tert-butylbenzene was also obtained in a similar yield.
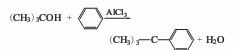
2. The isobutylene method consists of isobutylene and Benzene is obtained by hydrocarbonization in the presence of aluminum trichloride.
![]()
3. The preparation method is to first Cool benzene to 6°C, add anhydrous aluminum trichloride, and then slowly add the benzene solution of tert-butanol while stirring. Maintain the temperature at 8-11°C. After the addition, continue stirring for 4 hours. After the reaction is completed, suck out the benzene solution. Aluminum trichloride is destroyed with ice water. Extract with benzene, pour the benzene solution into ice water, combine the benzene solution, wash with water until neutral, distill benzene and low boiling matter first, then continue distillation, collect the 165-170°C fraction to get the finished product.
It is also possible to react isobutylene and benzene to produce tert-butylbenzene.
Place benzene in the reactor, add the catalyst aluminum trichloride, and then pass a measured amount of isobutylene from the bottom of the reactor within 1 to 1.5 hours. The reaction is exothermic, and the outside is cooled with cooling water. After the reaction is completed, add water to decompose the catalyst, transfer the liquid to a container and let it stand for layering, release the water layer, wash the organic layer with water, 5% sodium carbonate aqueous solution, and water in sequence until neutral, let it stand to separate the water layer, and beat the benzene layer. Enter the distillation kettle for distillation. Below 80°C is the benzene/water mixture, between 80 and 160°C is the benzene. By recycling, the temperature between 160 and 180°C is the tert-butylbenzene fraction, and the residue at the bottom of the still is polyalkylbenzene. When the input amount is 56 parts (mass), 19 parts of the product are obtained, the recovery amount is 37 parts, and the yield is 59%.
4. Prepared from benzene, isobutyl chloride and aluminum trichloride, or prepared by treating benzene and isobutanol with oleum.
Purpose
Intermediates for anti-allergic drugs, amphetamine and chlorpheniramine hydrochloride. Also used as polymer cross-linking agent and solvent.
���, the residue at the bottom of the still is polyalkylbenzene. When the input amount is 56 parts (mass), 19 parts of the product are obtained, the recovery amount is 37 parts, and the yield is 59%.
4. Prepared from benzene, isobutyl chloride and aluminum trichloride, or prepared by treating benzene and isobutanol with oleum.
Purpose
Intermediates for anti-allergic drugs, amphetamine and chlorpheniramine hydrochloride. Also used as polymer cross-linking agent and solvent.