Tri-n-butyltin hydride
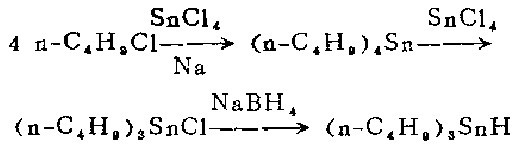
Structural formula
| Business number | 0799 |
|---|---|
| Molecular formula | C12H28Sn |
| Molecular weight | 291.06 |
| label |
Tributyltin hydride, Tri-n-butyltin hydrogen, Tributyl tin hydride, Tri-n-butyltin hydride, TBTH, Tri-n-butylstannane, Tributyltin hydride, [CH3(CH2)3]3SnH, reducing reagent |
Numbering system
CAS number:688-73-3
MDL number:MFCD00009416
EINECS number:211-704-4
RTECS number:WH8675000
BRN number:3587329
PubChem number:24889424
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25/4℃): 1.082
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): <0
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 1.473
8. Flash point (ºC): 40
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure ( kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: reacts with water to form tri-n-butyltin hydroxide. Can remain unchanged in dry state.
Toxicological data
1. Acute toxicity: mice (inhalation) LCLo: 1,460 mg/m3/10M Since the LD50 of table salt is 3,000 mg/kg, the acute toxicity of BPA is the same as that of table salt. 2.
Main irritating effects:
On the skin: irritation to the skin and mucous membranes
On the eyes: irritating effects
Sensitization : No known sensitizing effects.
Ecological data
Extremely harmful to water, even in small amounts. Do not let this product come into contact with groundwater, waterways and sewage systems. Even a small amount of this product seeping into groundwater will cause harm to drinking water.The water poses a danger and is also toxic to fish and plankton in the water. Highly toxic to organic matter in water. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 9
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 72.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Keep away from oxides, acids, halogens, air, heat and moisture.
2.When encountering water vapor, it generates tri-n-butyltin hydroxide. Can be stored in a dry state.
Storage method
1. Store in a container filled with dry inert gas and place in a cool, dry place. The storage area must be locked and the keys must be given to the technical experts and their assistants. Do not store it together with acidic substances, store it away from halogens, store it in the air, and store it away from oxidants and water sources. heat proof.
Synthesis method
1. Preparation of tetra-n-butyltin [2]
In a 1-liter three-neck flask equipped with a stirrer, reflux condenser and dropping funnel, Add sodium sand made of 8 moles of sodium and an appropriate amount of petroleum ether and heat until boiling. Under stirring, slowly add a mixture of 1 mole of anhydrous tin tetrachloride and 4 moles of 1-chlorobutane from the dropping funnel for about 30 minutes. The reaction begins quickly, external heating is discontinued, and the heat of reaction brings the mixture to boiling (45-70°C) within 0.5-1.5 hours.
After boiling, continue stirring for 4 to 6 hours, and then cool the reaction mixture to room temperature. Suction filter. Wash the filter cake (containing sodium chloride and a small amount of unreacted sodium) with petroleum ether. The filtrate was distilled under normal pressure to remove the solvent. The residue was crude tetra-n-butyltin, which was distilled under vacuum with a yield of 64%.
Tetra-n-butyltin can also be prepared by reacting n-butyl magnesium bromide with anhydrous tetra-n-butyl tin amide
2. Preparation of tri-n-butyl tin amide
Combine tetra-n-butyl tin and anhydrous tetra-n-butyl tin amide A mixture of 129 grams (58 ml) of anhydrous tin tetrachloride was heated to 220-230°C for 1.5 hours. Then cool, first to about 100°C in air and to 30°C in water. The cooling process takes approximately 45 minutes. Then heat to 220-230℃ for 1.5 hours. Filter the reaction mixture, distill the filtrate under vacuum, and collect the I40-152°C/10mm fraction to obtain 680g.
3. Production of tri-n-butyl hydrogenation
This step of the reaction is carried out in a fume hood under nitrogen flow. Place 19.2 grams (0.059 mol) of tri-n-butyltin chloride and 140 ml of ethylene glycol dimethyl ether <previous calcium hydride treatment fractionation) in a 250 ml constant pressure funnel and shake gently to make the tri-n-butyltin chloride completely Dissolve.
Also clamp a 1-liter three-neck flask equipped with a magnetic stirrer in an aluminum pot under a fume hood, then place the aluminum pot and flask on the electromagnetic stirrer, and install gas on the necks on both sides of the flask Inlet and outlet tubes. Oxygen-free dry nitrogen was continuously introduced during the reaction. The outer end of the outlet tube is connected to the mineral oil bubble meter. After nitrogen is introduced for a few minutes, 6.2 grams (0.16 mol) of powdered sodium borohydride is added from the middle mouth, and then 230 ml of pure ethylene glycol dimethyl ether is added. . Then install the dropping funnel filled with tri-n-butyltin chloride solution on the mouth of the middle bottle, add dry ice-acetone (or ice-salt) into the aluminum pot to keep the reactant at -11 to -10°C. Let cool for 30 minutes. Within 30 minutes with vigorous stirring, was dropped into the blue-n-butyltin chloride solution to form a white sodium chloride precipitate. After the dropwise addition, the reaction mixture was allowed to stand at -11 Let stand at 10°C for 10-15 minutes. Without filtration, the entire reaction mixture was transferred cold to a 1 liter round bottom flask under a stream of nitrogen. Connect the flask to the rotary evaporator. The well is immersed in a cold bath maintained at 0°C, and the receiving flask of the evaporator (1-liter round-bottom flask) is immersed in a dry ice-acetone bath at 80°C. Then connect the evaporator to the high vacuum system, and immerse it in a Dewar bottle containing liquid nitrogen through a -196°C vacuum trap), and continuously evacuate the reactants The evaporated by-product diborane was collected in a cold trap at 196°C. After the distillation is completed, the system is filled with nitrogen to normal pressure. Diborane is decomposed by the following method: rapidly introduce a nitrogen stream into a 196°C cold trap, and then enter an airtight cold trap (200-250 ml) and two airtight cold traps connected in series with it. Put 100-200 ml of acetone in a gas absorption bottle. Let this 196°C cold trap slowly heat up to room temperature, and diborane will be brought into acetone by the nitrogen flow. The generated diisopropoxyborane is decomposed into harmless boric acid and hydrogen using running water.
From rotary evaporationRemove the distillation bottle from above. Under nitrogen flow, extract the residue with three 25 ml portions of peroxide-free anhydrous ether (preferably evaporated to dryness). The ether extract was filtered through a dry sand core funnel under nitrogen flow, and the filtrate was evaporated at 0°C and below 1 mm for 30-40 minutes to remove the ether. Obtained 16.5 g (96%) of colorless liquid product
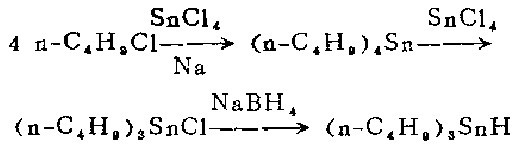
Purpose
1. Reducing reagent. Widely used in reductive cleavage and intramolecular cyclization.
2.Reducing agent. Reduction of alkyl halides to alkanes, acid halides to aldehydes, a-amino acid esters to carbonyl esters, selective reduction of geminal halogenated fluorinated cycloalkanes to fluorinated cycloalkanes. Alkenes and alcohols are converted into ethers, propylene alcohol is deoxygenated into alkenes, dihaloalkane is dehalogenated into alkenes, and thioacetals are desulfurized. Preparation of deoxysugar.