Triethyl orthoacetate Triethyl orthoacetate
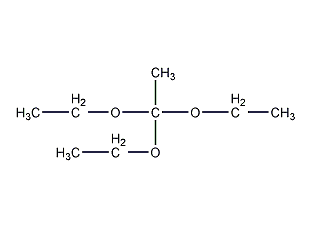
Structural formula
| Business number | 01N0 |
|---|---|
| Molecular formula | C8H18O3 |
| Molecular weight | 162 |
| label |
None |
Numbering system
CAS number:78-39-7
MDL number:MFCD00009223
EINECS number:201-112-4
RTECS number:None
BRN number:506201
PubChem number:24886849
Physical property data
1. Properties: colorless liquid with special odor
2. Density (g/mL, 25/4℃): 0.8847
3. Refractive index at room temperature (n20): 1.3968
4. Melting point (ºC): 74-78
5. Boiling point (ºC, normal pressure): 144~146℃
6. Boiling point (ºC, 5.2kPa): 47~45℃ (1.60kPa)
7. Refractive index: 1.3949
8. Flash point (ºC) :55℃
9. Specific optical rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11 . Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil and water (octanol/water) Log value of distribution coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Insoluble in cold water, miscible with ethanol, ether, acid, ester, chloroform and carbon tetrachloride.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 44.31
2. Molar volume (cm3/mol): 179.7
3. Isotonic specific volume (90.2K ): 404.5
4. Surface tension (dyne/cm): 25.6
5. Polarizability (10-24cm3): 17.56
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 76.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and Stability
Sensitive to water and needs to be stored dry.
Storage method
Keep dry.
Synthesis method
1. Prepared by passing a slight excess of dry hydrogen chloride into a mixture of anhydrous acetonitrile, anhydrous ethanol and anhydrous ether.
2. Preparation of iminomethyl acetate hydrochloride. In the cooled mixture of 135g anhydrous acetonitrile, 200ml absolute ethanol and 120ml absolute diethyl ether, add slightly more than molar amount of Dry hydrogen chloride. After sitting in the refrigerator overnight, the mixture solidifies into a white, glowing flaky mass. The ether was decanted and the hydrochloride salt was dried in a vacuum desiccator with soda lime for 24 hours to remove excess hydrogen chloride. Yield 86-95%. Methanol was used instead of ethanol to carry out this reaction, and white luminescent flakes of iminomethyl acetate hydrochloride were obtained with a yield of 71.6%.
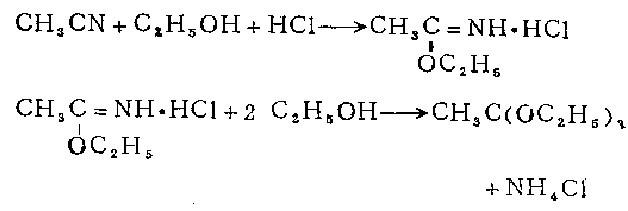
Preparation of triethyl orthoacetate Add 750 ml of absolute ethanol to 350 g of iminomethyl acetate hydrochloride that is absolutely dry and does not contain free hydrogen chloride. Put this mixture in a tightly stoppered bottle and keep it in a dry place for two weeks, shaking occasionally. Filter out the precipitated hydrogen chloride. These chlorinations must be retained because the reaction between ethanol and imine ether hydrochloride is extremely slow, so the solid precipitate still contains a large amount of unreacted hydrochloride. The filtrate is treated with 2 g of molten potassium carbonate to remove any traces of hydrogen chloride, and then fractionated under reduced pressure of 40 to 60 mm. The first fraction, 25-35°C, containing absolute acetate and some orthoacetate, can be reused for further treatment of the precipitated and new acetyl imine ethyl ether hydrochloride. The collected boiling point 55-57°C/50mm fraction is re-fractionated under normal pressure. The boiling point of pure triethyl orthoacetate is 144-146°C without decomposition. 750 ml of pure product was prepared in 4 times.
Purpose
1. Used in the preparation of trans-trisubstituted alkenes and chiral allenes and other olefinic preparations and as raw materials in the dye and pharmaceutical industries.
2. Triethyl orthoacetate [1] is stable under alkaline conditions and easy to react under acidic conditions. It can be hydrolyzed by acid catalysis to produce esters and esters under relatively mild conditions. alcohol. It hydrolyzes to produce ethyl acetate and ethanol, so these compounds will react with by-product water in the Fischer esterification reaction to produce low-boiling ethyl acetate and ethanol, thereby shifting the equilibrium and promoting the esterification reaction. Triethyl orthoacetate can induce the Claisen rearrangement of allyl alcohol, so it is often used to extend the carbon chain. In addition, it can be used in reactions to synthesize amides.
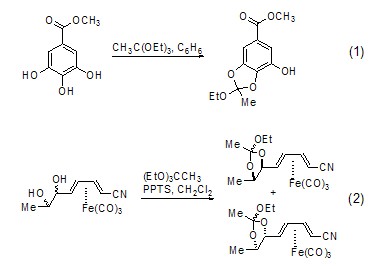
Synthesis of Acetate Under anhydrous conditions, some alcohols will also react with triethyl orthoacetate to form ether (Formula 1)[2]. Some metal complexes can also undergo this type of reaction (Formula 2)[3].
Claisen rearrangement Under acid catalysis, triethyl orthoacetate can react with allyl alcohol to form a C-C bond (formula 3)[4], which is essentially a Claisen rearrangement of alkoxy groups. This reaction may produce a variety of chiral isomers with certain spatial selectivity (Formula 4)[5].
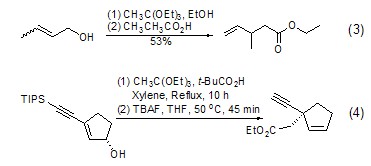
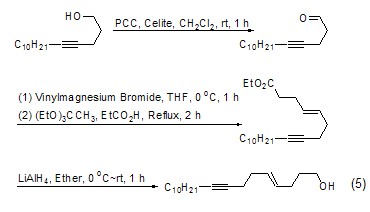
Carbon can be achieved through Claisen rearrangement chain growth. Since the rearrangement product is an ester, it can be further reduced to a hydroxyl group, thereby increasing the carbon chain (Formula 5)[6].
Allene GenerationTriethyl orthoacetate can react with α-substituted alkynes to generate esters with an allene structure (Formula 6)[7] .
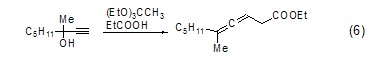
Carbon-carbon double Bond formationTriethyl orthoacetate can be used as a nucleophile to attack carbon atoms with lower electron cloud density to form carbon-carbon double bonds, such as its reaction with 1,3-malononitrile (Formula 7) [8].
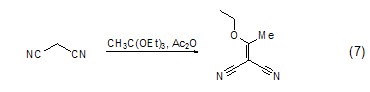
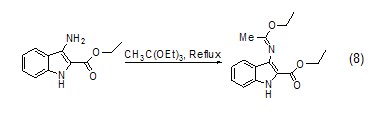
Formation of C-X bond Triethyl orthoacetate can also react with heteroatom groups such as amino groups to form C-N bonds or C=N double bonds (Formula 8)[9 ].
