Trimethyl orthoformate
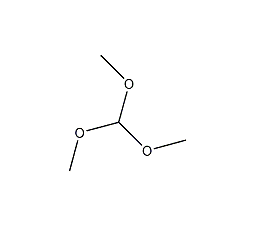
Structural formula
| Business number | 03XW |
|---|---|
| Molecular formula | C4H10O3 |
| Molecular weight | 106.12 |
| label |
Methyl orthoformate, Trimethylorthoformate, Trimethylformic acid, trimethoxymethane, Trimethoxymethane, paint dehydrating agent, aliphatic compounds |
Numbering system
CAS number:149-73-5
MDL number:MFCD00008483
EINECS number:205-745-7
RTECS number:RM6650000
BRN number:969215
PubChem number:24858434
Physical property data
1. Properties: colorless and transparent liquid with ester odor.
2. Density (g/mL, 20/4℃): 0.9676
3. Relative vapor density (g/mL, air=1): 3.67
4. Melting point (ºC): -53 ºC
5. Boiling point (ºC, normal pressure): 101
6. Flash point (ºC): 15 ºC
7. Saturated vapor pressure (kPa, 30ºC): 55.32
8. Explosion upper limit (%, V/V): 5.1
9. Explosion lower limit (%, V/V): 1.4
10. Solubility: soluble in water, alcohol and ether.
11. Refractive index (20ºC): 1.3793
12. Relative density (25℃, 4℃): 0.9623
13. Refractive index at room temperature (n 25): 1.3773
14. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2471.2
15. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -531.9
16. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2433.1
17. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -570.0
Toxicological data
Rabbit transdermal: 500mg/24 hours, mild stimulation.
Rabbit eye: 100mg/24 hours, moderate irritation.
Irritating to eyes and skin.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 25.77
2. Molar volume (cm3/mol): 114.0
3. Isotonic specific volume (90.2K ): 247.6
4. Surface tension (dyne/cm): 22.2
5. Polarizability (10-24cm3):10.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 28.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure. Highly flammable.
Incompatible materials: strong oxidants, acids
2. This product is an irritant. The oral LD50 in rats is 3130mg/kg. It is irritating to the skin, eyes and mucous membranes. effect.
3. It is very sensitive to moisture, flammable, has strong volatility and pungent odor, and should be used in a fume hood.
Storage method
Store sealed in a dry and cool place.
This product is packaged in a 200kg galvanized steel drum or glass bottle with a wooden box or calcium plastic box reinforced with padding. Store in a cool, ventilated warehouse away from fire sources and open flames; store and transport isolated from oxidants and avoid sunlight exposure.
Synthesis method
It is obtained by reacting chloroform and methanol in the presence of caustic soda (or chloroform and sodium methoxide).
![]()
Purpose
1. In the pharmaceutical industry, it is used in the production of vitamin B1, vitamin A, sulfadiazine, pipemidic acid and antibacterial agents. Among them, methyl orthoformate accounts for the largest proportion of vitamin B1 consumption. Also used in the dye and fragrance industry. In coatings, it is used as a dehydrating agent to prevent polyurethane or epoxy coatings from hardening due to hydration.
2. Used as an intermediate in the production of vitamin B1, sulfonamide drugs, antibiotics and other drugs, as a raw material for spices and pesticides and as an additive for polyurethane coatings.
3. Trimethyl orthoformate is a commonly used alkylating reagent and formylating reagent in chemical laboratories. They are easy to store but highly reactive. They can be used as alkylating reagents and can easily transfer alkyl groups to various alcohol hydroxyl groups; when used as formylating reagents, they can be used under acidic or alkaline conditions.
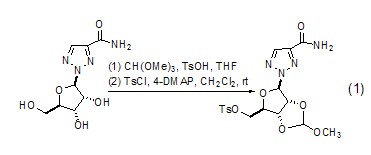
Alkylation reaction with alcohol When trimethyl orthoformate is used as an alkylation reagent, it can cause corresponding alkylation reactions of various alcohols. Alkylation reaction of polyols can occur to generate the corresponding cyclic orthoformate (Formula 1)[1~3].
Aldehydes or ketones Protection Orthoformate can be used to protect aldehydes and ketones to generate formate and the corresponding acetal or ketal (formula 2)[4,5].
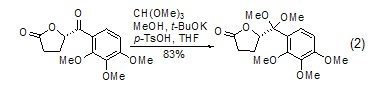
Formylation reaction Under the catalysis of Lewis acid, orthoformate can be used as a formylation reagent. Compared with other formylation reagents, it has the characteristics of easy operation and higher yield. Under acid catalysis, orthoformate can easily generate dialkoxy carbonium ions in situ and can be directly used in the formylation reaction. This type of formylation reaction can be on the α-C ortho to the carbonyl group (Formula 3)[6].
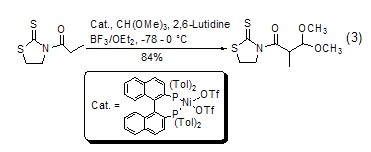
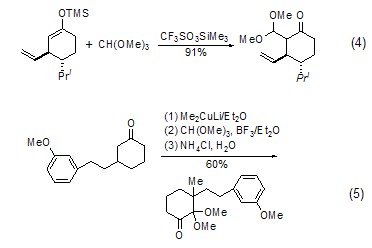
With enol ( or analogues) reaction The silyl ether of enol (formula 4)[7] or the enolate of ketone (formula 5) [8] can be reacted with The carbonium ion generated in situ by orthoformate reacts to generateβ-acetal ketone compounds.
