Trimethylphosphate
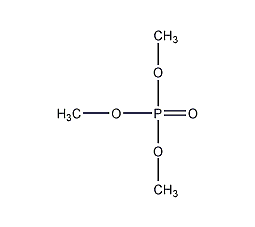
Structural formula
| Business number | 05AH |
|---|---|
| Molecular formula | C3H9O4P |
| Molecular weight | 140.08 |
| label |
Trimethylphosphate, trimethyl phosphate, O,O,O-Trimethyl phosphate, Trimethoxyphosphine oxide, Extracting agent, textile oil, Anti-staining agent for polymers |
Numbering system
CAS number:512-56-1
MDL number:MFCD00008348
EINECS number:208-144-8
RTECS number:TC8225000
BRN number:1071731
PubChem number:24854454
Physical property data
1. Properties: colorless transparent liquid
2. Density (g/ cm3, 20/4℃): 1.2144
3. Relative vapor density (g/cm3, air=1): Undetermined
4. Melting point (ºC): -70
5. Boiling point ( ºC, normal pressure): 197.2
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index (20ºC): 1.3967
8. Flash point (ºC): 148.9
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol ): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (polymer) Log value of the partition coefficient (alcohol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V ): Undetermined
19. Solubility: miscible with various resins, gums, and organic solvents, and decomposed when dissolved in water.
Toxicological data
Short-term exposure affects the central nervous system, and long-term exposure can cause weakness and paralysis. Causes genetic damage in humans. The oral LD50 in rats is 1.65g/kg.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 28.05
2. Molar volume (cm3/mol): 121.2
3. Isotonic specific volume (90.2K ): 282.6
4. Surface tension (dyne/cm): 29.4
5. Polarizability (10-24cm3):11.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 44.8
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 82.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Easily soluble in water, soluble in ether, and insoluble in ethanol. Low toxicity, irritating to skin and mucous membranes. It is non-flammable and decomposes when heated to produce toxic fumes of phosphorus oxide.
2. Rat oral LD501.65g/kg. It mainly damages the central nervous system and may cause flaccid or spastic paralysis. The production operation site should be ventilated, production equipment should be prevented from leaking, and operators should wear protective equipment.
Storage method
Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
This product is packed in iron drums with a specification of 200kg. Store in a cool, dry and ventilated place. Fireproof. Store and transport according to regulations on toxic, flammable and dangerous goods.
Synthesis method
1. Phosphorus oxychloride reacts with methanol in the presence of potassium carbonate to form trimethyl phosphate. At the same time, the reaction produces dimethyl phosphate potassium salt, and dimethyl sulfate is used to react to produce trimethyl phosphate. The crude trimethyl phosphate product is washed with water, decolorized, dehydrated, and distilled under reduced pressure to obtain the finished product. Raw material consumption quota: phosphorus oxychloride 1094kg/t, methanol 686kg/t.

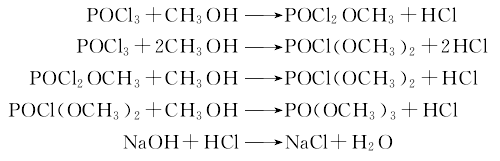
2.Add methanol and potassium carbonate into the reaction pot, cool to 5°C, start adding phosphorus oxychloride dropwise, and control the temperature below 30°C. After 2 hours of dripping, stir for another 0.5 hours. The pH value Control it at 7 to 8; then add dimethyl sulfate, reflux for 3 hours and recover methanol. Then cool the material in the pot to below 20°C, add carbon tetrachloride to filter, wash the filter cake with a small amount of carbon tetrachloride, and wash the washing liquid with The filtrates are combined, carbon tetrachloride is recovered, and the crude product is obtained by distillation under reduced pressure. Add distilled water and activated carbon to the crude product, filter and add anhydrous potassium carbonate to dehydrate, and finally distill under reduced pressure to obtain the product. The main reaction is as follows:
Purpose
1. Mainly used as solvents and extractants for medicines and pesticides. Pesticide solvents are mainly used as anti-coloring agents for textile oils and polymers in Japan.
2.It is used as additive flame retardant and plasticizer, but the flame retardant efficiency is not high and the volatility is high. Generally Cooperates with other flame retardants.