triphenylmethane chloride

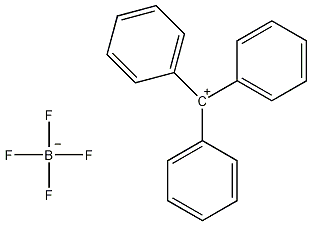
Structural formula
| Business number | 04AP |
|---|---|
| Molecular formula | C19H15BF4 |
| Molecular weight | 330.13 |
| label |
Trityltetrafluoroborate, triphenyltetrafluoroborate carbon, triphenyl-methyliutetrafluoroborate(1-), Tritylium tetrafluoroborate, Trityl fluoroborate, Triphenylcarbenium tetrafluoroborate, Triphenylmethyl tetrafluoroborate, Trityl tetrafluoroborate, Triphenylcarbeniumtetrafluoroborate,98%, Methylium, triphenyl-, tetraflu |
Numbering system
CAS number:341-02-6
MDL number:MFCD00013120
EINECS number:206-433-3
RTECS number:None
BRN number:5085649
PubChem number:24850086
Physical property data
1. Physical property data
Character: yellow solid
Density (g/mL, 25/4℃ ): Not available
Relative vapor density (g/mL, air=1): Not available
Melting point (ºC): 205- 215
Boiling point (ºC, normal pressure): Not available
Boiling point (ºC, 5.2kPa): Not available
Refractive index: Not available
Flash point (ºC): Not available
Specific rotation (º) : Not available
Autoignition point or ignition temperature (ºC): Not available
Vapor pressure (kPa, 25ºC): Not available
Saturated vapor pressure (kPa, 60ºC): Not available
Heat of combustion (KJ/mol): Not available
Critical temperature (ºC): Not available
Critical pressure (KPa): Not available
Oil and water (octanol /water) logarithmic value of the distribution coefficient: not available
Explosion upper limit (%, V/V): not available
Lower explosion limit ( %, V/V): Not available
Solubility: Not available
Toxicological data
2. Toxicological data:
Acute toxicity: Not available.
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 24
8. Surface charge: 0
9. Complexity: 215
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
Store at 2-8℃.
Synthesis method
1. In a 250 ml Erlenmeyer flask equipped with a magnetic stirrer and a nitrogen introduction tube, place 27.9 g (0.1 mol) of triphenyl chloride and 100 ml Thousand dry benzene. The mixture was stirred under dry nitrogen until a clear solution formed. Cool the solution to 0°C in an ice-salt bath. Add 13.4 grams (0.1 mol) of anhydrous monodimethyl ether tetrafluoroborate complex through the dropping funnel within 10 minutes, and continue stirring for 1 minute without further cooling. 50 ml of petroleum ether was added to the reaction mixture, and filtered through a glass core funnel. The bright yellow solid was washed with two 50 ml portions of petroleum ether, dried under a stream of dry nitrogen and then in vacuo. Get 2 grams (73%, melting point is about 200°C (decomposition).

Purpose
1. Same as trityl perchlorate, but more stable, so it is used more often. Alcohol oxidizing agent. Synthesis of alkenes.