Tetra-n-butylammonium fluoride

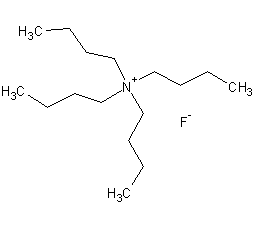
Structural formula
| Business number | 04SU |
|---|---|
| Molecular formula | C16H38FNO |
| Molecular weight | 261.47 |
| label |
Tetra-n-butyl-ammonium fluoride, Tetra-n-butylammonium fluoride, tetrahydrofuran solution, Tetrabutylammonium fluoride, Tetrabutylamine fluoride, Tetrabutylammonium fluoride, Tetra-n-butylammonium fluoride, 1M SOLN. IN THF, Tetra-n-butylammonium fluoride, 75% W/W AQ. SOLN., Tbaf, Tetranutylammonium fluoride hydrate, 1-Butanaminium,N,N,N-tributyl-,fluoride, n,n,n-tributyl-1-butanaminiufluoride, TBAF solution, Tetrabutylammonium fluoride solution, TetranbutylammoniumfluorideMsolninTHF, Tetrabutylammonium fluoride 1.0M solution in THF, Strong base reagent |
Numbering system
CAS number:429-41-4
MDL number:MFCD00011747
EINECS number:207-057-2
RTECS number:None
BRN number:3570522
PubChem number:24860012
Physical property data
1. Properties: white solid, easily hygroscopic.
2. Density (g/mL, 25/4℃): 0.953
3. Relative vapor density (g/mL, air =1): Not available
4. Melting point (ºC): 62-63
5. Boiling point (ºC, normal pressure): Not available
6. Boiling point (ºC, 5.2kPa): Not available
7. Refractive index: 1.456
8. Flash point (ºC): Not available
9. Specific rotation (º): Not available
10. Autoignition point or ignition temperature (ºC): Not available
11. Vapor pressure (kPa, 25ºC): Not available
12. Saturated vapor pressure (kPa, 60ºC): Not available
13. Heat of combustion (KJ/mol): Not available
14. Critical temperature (ºC): Not available
15. Critical pressure (KPa): Not available
16. Oil and water (octanol /water) logarithmic value of the distribution coefficient: not available
17. Explosion upper limit (%, V/V): not available
18 .Lower explosion limit (%, V/V): Not available
19. Solubility: soluble in water, acetonitrile, THF.
Toxicological data
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 116
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
This reagent must be handled in a fume hood.
Storage method
None
Synthesis method
1. Prepared by reacting hydrofluoric acid aqueous solution and tetrabutylammonium bromide aqueous solution in ion exchange resin. After the reaction is completed, the resin is washed repeatedly with water, and the water in the eluent is evaporated to dryness to obtain an oily product.
2. Slowly add hydrofluoric acid aqueous solution (10% W/V) to 100 ml of tetra-n-butylammonium hydroxide aqueous solution until the pH begins to drop. Use dilute hydrofluoric acid (3%) Titrate to pH 7 and dilute the light brown solution to 300 ml (about 0.5M) with water. Cool in ice to precipitate fine crystals of caged (n-C4H9)4NF.32.8. Filter, wash with ice water, and finally dry in the air. . Obtain 72 grams. Concentrate the mother liquor, dilute to about 0.5M and cool to 0°C. You can also obtain a second portion of 32 grams) clathrate compound. The clathrate can be stored in polyolefin containers at 0°C for several weeks.
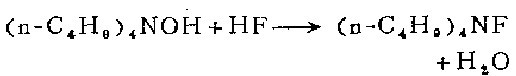
3. Add 25 ml of industrial grade 10% tetra-n-butyl hydroxide was titrated with dilute hydrofluoric acid to pH 7-8.5. The solution was cooled to 10°C to form a white clathrate compound. The liquid was filtered off and the clathrate was washed twice with cold water. Subtract most of the water under water pump suction. The resulting paste is dried at 30-40°C/0.5mml and under the action of phosphorus pentoxide for 15-20 hours. Anhydrous tetrabutyl fluoride is stored over phosphorus pentoxide.
Purpose
1. Cleave the protecting group. Catalyst for silylation and elimination reactions. Preparation of selenoaldehydes and thioaldehydes.
2. Strong alkaline reagent. Fluorinating agents. Alcohol silylation catalyst. Split silane ethers. Deprotection of phosphate protecting groups. Alkaline reagent in Aldol condensation, alkylation reaction, hydroxyacylation reaction, etc.
3. Tetrabutylammonium fluoride (TBAF)[1] can be dissociated into (n-Bu) during the reaction 4N+ and F– ions. It is a commonly used fluorinated reagent that can replace nitro, halogen and other groups, but its substitution performance is weaker than that of cyano. It is also a commonly used desilylation reagent.
Fluorine substitution reaction TBAF can be used for most fluorine substitution reactions. For example: 2-fluoro-4-bromo-pyridine is prepared from 2-nitro-4-bromo-pyridine (Formula 1)[2]. Mild reaction conditions and high reaction yield are the main reasons for using TBAF as the fluorinated reagent.
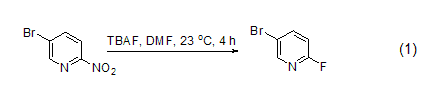
Desilylation reaction TBAF is widely used in desilylation reactions. This type of reaction can usually be carried out under relatively mild conditions and has a high yield. It is the key to cutting off Si-O (Formula 2)[3] and Si-N are also applicable to heteroatoms (formula 3) [4] and carboxyl groups (formula 4) [5] Desilylation reaction.

TBAF is still a very strong Dealkylosilation reagent, widely used to cut Si-C bonds, especially sp2-C-Si and sp3-C adjacent to double or triple bonds. -Si bond (Formula 5)[6]. If TBAF is used in combination with a transition metal catalyst, the desilylation reaction and the coupling reaction can be carried out simultaneously (Formula 6)[7].

Catalytic nucleophilic substitution Reaction TBAF can catalyze nucleophilic substitution reactions on dinitrobenzene, such as the reaction between p-dinitrophenyl and trifluoroethanol (Formula 7)[8]. The catalytic mechanism is to first replace a nitro group with fluorine, and then the hydroxyl group of trifluoroethanol nucleophilically attacks the carbon atom connected to the fluorine to obtain the target product.

