Tetradecanol Tetradecanol
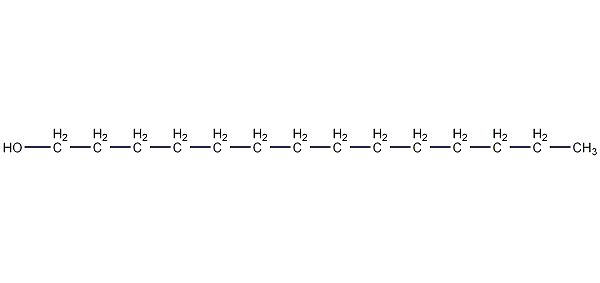
Structural formula
| Business number | 036H |
|---|---|
| Molecular formula | C14H30O |
| Molecular weight | 214.39 |
| label |
Myristol, 1-tetradecanol, Tetradecyl alcohol, Myristol, 1-Tetradecanol, n-Tetradecyl alcohol, Myristyl alcohol, Surfactant, detergent, alcohol solvents, Cream softener |
Numbering system
CAS number:112-72-1
MDL number:MFCD00004757
EINECS number:204-000-3
RTECS number:XB8655000
BRN number:1742652
PubChem number:24888721
Physical property data
1. Properties: Colorless to white waxy solid flakes with a waxy odor.
2. Relative density (g/mL, 38/4ºC): 0.8346
3. Relative vapor density (g/mL, air=1): 7.4
4. Melting point (ºC): 37.8
5. Boiling point (ºC, normal pressure): 287
6. Boiling point (ºC, 2.0Kpa): 167
7. Flash point (ºC): 145
8. Autoignition point or ignition temperature (ºC): 240
9. Solubility: soluble in ether, easily soluble In ethanol, insoluble in water. Dissolves 0.02% in water at 20°C.
10. Refractive index at room temperature (n25): 1.432560
11. Relative density (25℃, 4℃ ): 0.822740
12. Relative density (20℃, 4℃): 0.836d
13. van der Waals area (cm2·mol-1): 2.113×1010
14. van der Waals volume ( cm3·mol-1): 154.700
15. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -9269.2
16. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -527.4
17. Liquid phase Standard heat of combustion (enthalpy) (kJ·mol-1): -9216.0
18. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -580.6
19. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -9167.0
20 .Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -629.6
Toxicological data
Acute toxicity: rat oral LD50: >5000mg/kg; rabbit dermal LD50: >5000mg/kg
Ecological data
Mildly hazardous to water.
Molecular structure data
1. Molar refractive index: 68.44
2. Molar volume (cm3/mol): 257.1
3. Isotonic specific volume (90.2K): 605.8
4. Surface tension (dyne/cm): 30.8
5. Polarizability (10-24cm3): 27.13
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 102
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants, acidic chlorides, and acid anhydrides. It has the chemical reactivity of higher primary alcohols.
2. Exist in smoke.
Storage method
Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging must be sealed and protected from moisture. They should be stored separately from oxidants, acidic chlorides, and acid anhydrides, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The mixed alcohol is obtained by methyl esterification and high-pressure hydrogenation of coconut oil, and then the product is obtained by fractional distillation. It can also be produced by the Ziegler method of ethylene oligomerization, oxidation and hydrolysis.
It can be isolated from spermaceti and whale oil or catalytically reduced with their corresponding acids and esters. Depending on the conditions and prices of industrial production, the application of high-pressure hydrogenation or lithium-aluminum hydride reduction should be considered. .
Purpose
Myristol is a modifier in the special fragrance of daily chemical fragrances to add a waxy aroma. In addition, it is also an emulsifier, surfactant and thickener, so this compound is actually used in the fragrance industry and daily chemical industry. Related raw materials. Used in the manufacture of tetradecyl mercaptan and detergents, etc. It can be used as raw material for organic synthesis and surfactants. Can also be used as a cream softener.
