Tetrahydrofuran Tetrahydrofuran

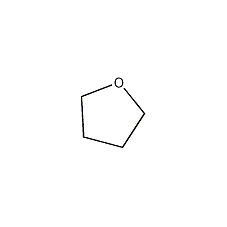
Structural formula
| Business number | 030B |
|---|---|
| Molecular formula | C4H8O |
| Molecular weight | 72.11 |
| label |
oxolanes, 1 oxy 5 ring, tetramethylene oxide, Tetramethylene oxide, THF, chromatography reagents, synthetic raw materials |
Numbering system
CAS number:109-99-9
MDL number:MFCD00005356
EINECS number:203-786-5
RTECS number:LU5950000
BRN number:102391
PubChem number:24864878
Physical property data
1. Properties: Colorless and volatile liquid with an ether-like odor. [1]
2. pH value: 5 (20% aqueous solution) [2]
3. Melting point (℃ ): -108.5[3]
4. Boiling point (℃): 66[4]
5. Relative density (Water=1): 0.89[5]
6. Relative vapor density (air=1): 2.5[6]
7. Saturated vapor pressure (kPa): 19.3 (20°C) [7]
8. Heat of combustion (kJ/mol): -2515.2[ 8]
9. Critical temperature (℃): 268[9]
10. Critical pressure (MPa): 5.19[10]
11. Octanol/water partition coefficient: 0.46[11]
12. Flash point (℃): -14 (CC); -20 (OC) [12]
13. Ignition temperature (℃): 321[13]
14. Explosion upper limit (%): 11.8[14]
15. Explosion lower limit (%): 1.8[15]
16. Solubility: Soluble in most organic solvents such as water, ethanol, ether, acetone, and benzene. [16]
17. Refractive index (n20ºC): 1.4050
18. Refractive index (n25ºC): 1.4040
19 . Viscosity (mPa·s, 0ºC): 0.66
20. Viscosity (mPa·s, 20ºC): 0.55
21. Viscosity (mPa·s, 30ºC): 0.47
22. Viscosity (mPa·s, 40ºC): 0.42
23. Heat of evaporation (KJ/kg, 66ºC): 410
24. Specific heat capacity (KJ /(kg·K), 20ºC, constant pressure, liquid): 1.96
25. Specific heat capacity (KJ/(kg·K), 60ºC, constant pressure, gas): 1.55
26. Critical density (g·cm-3): 0.322
27. Critical volume (cm3·mol-1): 224
28. Critical compression factor: 0.259
29. Eccentricity factor: 0.226
30. Solubility parameter (J·cm-3)0.5: 19.129
31. van der Waals area (cm2·mol-1): 6.000×109
32. van der Waals volume (cm3·mol-1 ): 44.620
33. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2533.16
34. Gas phase standard claimed heat ( Enthalpy) (kJ·mol-1): -184.18
35. Gas phase standard entropy (J·mol-1·K– 1): 302.41
36. Gas phase standard formation free energy (kJ·mol-1): -81.1
37. Gas phase standard Hot melt (J·mol-1·K-1): 76.25
38. Liquid phase standard combustion heat (enthalpy) (kJ·mol -1):-2501.07
39. Liquid�Furfural is mainly produced by hydrolysis of agricultural and sideline products such as corn cobs. This method is seriously polluting and is not conducive to large-scale production, so it has been gradually eliminated. 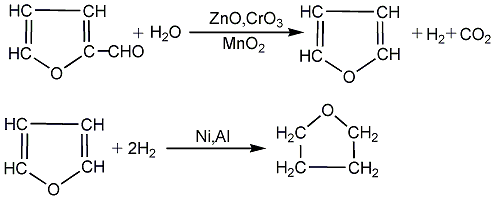
(2) Maleic anhydride catalytic hydrogenation method
Maleic anhydride and hydrogen enter the reactor equipped with a nickel catalyst from the bottom. The ratio of tetrahydrofuran and γ-butyrolactone in the product can be controlled by adjusting the operating parameters. The reaction product and raw material hydrogen are cooled to about 50°C and enter the bottom of the washing tower to separate the unreacted hydrogen and gaseous products from the liquid products. The unreacted hydrogen and gaseous products are washed and recycled to the reactor. The liquid products are distilled to obtain tetrahydrofuran products. .
This process can arbitrarily adjust the ratio of γ-butyrolactone to tetrahydrofuran within the range of 0 to (5:1). The single-pass conversion rate of maleic anhydride reaches 100%, and the selectivity of tetrahydrofuran is 85% to 95%. The product The content reaches 99.97%. This process has the characteristics of good catalyst performance, simple process and low investment. 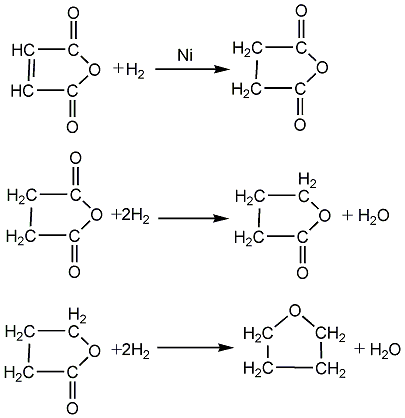
(3) 1,4-butanediol dehydration cyclization method
The process is as follows: add 1087kg of 22% sulfuric acid aqueous solution into the reactor, add 1,4-butanediol at a rate of 110kg/h at 100°C, maintain the top temperature at 80°C, and add 1,4-butanediol at a rate of about 110kg at 100°C. /h to obtain an aqueous solution containing 80% tetrahydrofuran from the top of the tower. After adding 50t of 1,4-butanediol, approximately 70kg of coke mass was removed from the reactor. The coke is filtered, and the obtained sulfuric acid aqueous solution can be reused. The yield of tetrahydrofuran in this process can reach more than 99%.
Sulfuric acid is the earliest catalyst used in the industrial production of tetrahydrofuran, and it is also the most commonly used catalyst in today’s production. This process has mature technology, relatively simple process, low reaction temperature, and high tetrahydrofuran yield. However, sulfuric acid can easily corrode equipment and pollute the environment. 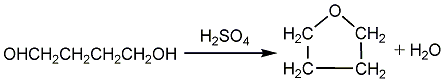
(4) Dichlorobutene method
With 1 , 4-dichlorobutene is used as raw material, which is hydrolyzed to form butene diol, which is then obtained by catalytic hydrogenation.
1,4-Dichlorobutene is hydrolyzed in a sodium hydroxide solution to produce butenediol at 110°C. The sodium chloride is removed by centrifugation. The filtrate is concentrated in an evaporative crystallizer to separate the alkali metal carboxylic acid. salt, and then remove high boilers in the distillation tower. The refined butylene glycol is fed into the reactor, and nickel is used as the catalyst. At 80 to 120°C and a certain pressure, butylene glycol is hydrogenated to form butylene glycol. After distillation, it is fed into the cyclization reactor and processed under normal pressure. And generate crude tetrahydrofuran in an acidic medium at 120-140°C, distill it to dehydrate and remove high boiling matter, and finally distill it to obtain high-purity tetrahydrofuran.
This method is easy to operate, has mild conditions, high yield, small amount of catalyst, and can be used continuously.
(5) Butadiene oxidation method
Uses butadiene as raw material, oxidizes it to obtain furan, and then hydrogenates it. This method has been industrialized abroad.
(6) Industrial production first used furfural as raw material, and the mixture of furfural and steam was passed into a reactor filled with zinc-chromium-manganese metal oxide (or palladium) catalyst. The carbonyl group is removed at 80-120°C to form furan; then, using skeleton nickel as a catalyst, furan is hydrogenated at 80-120°C to obtain tetrahydrofuran. This method produces 1 ton of tetrahydrofuran, which consumes approximately 3 tons of polysaccharide. There are many production methods developed later. The industrialized method includes the 1,4-butanediol catalytic dehydration cyclization method. Because butanediol is produced from acetylene and formaldehyde, this method is called the Reppe method; using The production of tetrahydrofuran from 1,4-dichlorobutene, a by-product of chloroprene monomer chloroprene, is called the dichloroprene method; in recent years, a catalytic hydrogenation method using maleic anhydride as raw material has been developed.
(7) In the presence of zinc oxide, chromium oxide, and manganese dioxide, furfural can be catalytically deformed, and hydrogenated in the presence of nickel-aluminum catalyst to form tetrahydrofuran:

Purpose
1. This product is an organic solvent with excellent performance. It is widely used due to its fast dissolution speed and good penetration and diffusion into the surface and interior of the resin. Used as solvent in Grignard reaction, polymerization reaction, LiAlH4 reduction condensation reaction, and esterification reaction. By dissolving polyvinyl chloride, polyvinylidene chloride and their copolymers, low-viscosity solutions can be obtained, which are often used in the manufacture of surface coatings, protective coatings, adhesives and films. It is also used in inks, paint removers, extraction agents, artificial leather surface treatment, etc. This product’s self-polymerization and copolymerization can produce polyether-type polyurethane elastomer, which is characterized by high resilience performance. This product is an important chemical raw material and can be used to prepare butadiene, nylon, polybutylene glycol ether, γ-butyrolactone, polyvinylpyrrolidone, tetrahydrothiophene, etc. This product is also an intermediate in organic synthesis of drugs and other substances.
2. Tetrahydrofuran is an important raw material for organic synthesis and an excellent solvent. Tetrahydrofuran has good solubility for many organic compounds. It can dissolve all organic compounds except polyethylene, polypropylene and fluororesin. , especially has good dissolving effect on polyvinyl chloride, polyvinylidene chloride and butylaniline, and is widely used as a reactive solvent. As a common solvent, tetrahydrofuran has been widely used in surface coatings, protective coatings, inks, extraction agents and surface treatment of artificial leather. Tetrahydrofuran is an important raw material for the production of polytetramethylene ether glycol (PTMEEG) and the main solvent in the pharmaceutical industry. Used as a solvent for natural and synthetic resins (especially vinyl resins), and also used in the production of butadiene, adiponitrile, adipic acid, hexamethylenediamine, etc.
3. Used as solvents, chemical synthesis intermediates, and analytical reagents. [28]
(especially vinyl resin) solvent, also used to make butadiene, adiponitrile, adipic acid, hexamethylenediamine, etc.
3. Used as solvents, chemical synthesis intermediates, and analytical reagents. [28]
