Tetramethylammonium hydroxide Tetramethylammonium hydroxide
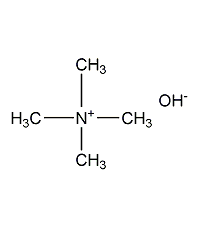

Structural formula
| Business number | 01JU |
|---|---|
| Molecular formula | C4H13NO |
| Molecular weight | 91.05 |
| label |
N,N,N-Trimethylmethanaminium Hydroxide, (CH3)4N(OH) |
Numbering system
CAS number:75-59-2
MDL number:MFCD00008280
EINECS number:200-882-9
RTECS number:PA0875000
BRN number:3558708
PubChem number:24866695
Physical property data
1. Properties: It has a certain ammonia smell, is highly alkaline, and easily absorbs CO2 in the air. It is usually made into 10% or 25% aqueous solution. Tetramethylammonium hydroxide containing 5 molecules of crystal water is colorless. Deliquescent needle crystals. When heated to the boiling point, it easily decomposes into trimethylamine and methanol, with a specific gravity of 1.00 (25/4°C).
2. Density (g/mL, 25/4℃): 1.016
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 62-71°C.
5. Boiling point (ºC, normal pressure): 120℃
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: 1.3806
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition Temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Soluble in water, ethanol, etc.
Toxicological data
1. Acute toxicity
Mouse subcutaneous LD50: 19mg/kg
Rabbit intravenous LD50: 1mg/kg
Pig skin LD50: 25mg/kg
Frog gastrointestinal LDL0: 5mg/kg
Frog LDL0: 1515 ug/kg
2
Main irritant effects:
On skin: Causes corrosive effects on skin and mucous membranes.
On eyes: Strong corrosive effects
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large quantities of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Hazardous to organic matter in water
�Excessive discharge into rivers and sewers can cause an increase in ph value. Excessively high ph value is harmful to organic matter in the water.
Concentration dilution during use can greatly reduce the ph value, thereby reducing Hazards to water caused by product discharge
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 19.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
None
Purpose
In terms of silicone, it can be used as catalysts for dimethyl silicone oil, benzyl silicone oil, silicone diffusion pump oil, solvent-free silicone molding compound, silicone resin, silicone rubber, etc. In terms of analysis, it is used as a polarographic reagent and in product purification as an ashless alkali to precipitate many metal elements. In the production of organic silicon wafers, it is often used as a brightener, cleaning agent and contact agent for computer silicon wafer surfaces. This product is a catalyst in organosilicon polymerization. Its advantages are: it is strongly alkaline and stable at temperatures that do not exceed the decomposition point. When it exceeds the decomposition point, it quickly decomposes into trimethylamine and methanol. When the catalysis is completed, it is easy to remove without leaving any residue. No pollution to silicone products. Therefore, it is also called a “temporary catalyst”. It can be used in polarographic tests and to precipitate hydrides of many elements that do not contain ash.
