Tetramethylthiuram disulfide

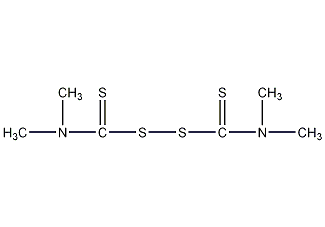
Structural formula
| Business number | 03RF |
|---|---|
| Molecular formula | C6H12N2S4 |
| Molecular weight | 240.43 |
| label |
N,N’-Tetramethyldisulfidecarbonylamine, accelerator T, accelerator TMTD, Accelerator TMTD-II, accelerator TT, Bis(thiocarbonyldimethylamine) disulfide, Tetramethylthiuram disulfide, Thiram, Aapirol,Arasan,Falitiram,Fernide,Formalsol, Fungicide |
Numbering system
CAS number:137-26-8
MDL number:MFCD00008325
EINECS number:205-286-2
RTECS number:JO1400000
BRN number:1725821
PubChem number:24889011
Physical property data
1. Properties: white crystalline powder
2. Relative density: 1.29
3. Melting point (℃): 155~156
4. Solubility: insoluble in water, insoluble in dilute caustic alkali, gasoline, slightly soluble in ethanol, ether, soluble in benzene, acetone, chloroform, carbon tetrachloride , carbon disulfide, dichloroethane.
Toxicological data
1. Acute toxicity: Oral – rat LD50: 560 mg/kg; Oral – mouse LC50: 1250 mg/kg.
2. Irritation data Eyes – Rabbit 100 mg/24 hours Moderate.
3. Irritating to respiratory tract and skin.
The acute oral LD50 in rats is 780 ~ 865mg/kg. The acute oral LC50 in mice is 1500~2000mg/kg. LC50 is 4mg/L
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 67.86
2. Molar volume (cm3/mol): 179.9
3. Isotonic specific volume (90.2K): 516.6
4. Surface tension (3.0 dyne/cm): 67.8
5. Polarizability (0.5 10-24cm3): 26.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 121
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 158
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and Stability��
1. Soluble in benzene, acetone, chloroform, carbon disulfide, slightly soluble in ethanol, ether and carbon tetrachloride, insoluble in water, dilute alkali and gasoline. Heat with water to generate dimethylamine and disulfide.
2. Decomposed when exposed to acid. The industrial product is white or light yellow powder. It has an irritating effect on human mucous membranes and skin. People who have been exposed to it for a long time may have allergic reactions after drinking alcohol.
Storage method
Store in a ventilated and cool place, avoid direct sunlight.
Synthesis method
1. Preparation of sodium dimethyldithiocarbamate. Add carbon disulfide and dimethylamine to 15% sodium hydroxide solution. Control the reaction temperature at 40 to 45°C while stirring. The condensation reaction lasts for more than 1 hour. When the pH When the value is 9 to 10, the reaction is complete and sodium dimethyldithiocarbamate is generated.

2. Preparation of accelerator TMTD in the condensation product Add 10% sodium nitrite aqueous solution, stir and dissolve, filter, add to the oxidation reactor, slowly drop sulfuric acid, introduce air, control the temperature at about 8°C, carry out the oxidation reaction, and generate the accelerator TMTD. The accelerator TMTD precipitates as a solid in the reaction system. After suction filtration, water washing, dehydration, drying and screening, the finished product is obtained. 
3.Add dimethylamine and the catalyst previously dissolved in the solvent to the reaction kettle, add carbon disulfide to the solvent, heat to a suitable temperature, and introduce oxygen while stirring. After the reaction is complete for a certain period of time, the finished product is obtained through filtration, washing and drying.
4.Add 9g (0.2mol) dimethylamine and 0.23g catalyst into the three-necked flask and stir below 20°C. Add 18.04g (0.24mol) carbon disulfide dropwise. After continuing to stir, add 26.8g (0.2mol) 30% sodium hydroxide dropwise. At this time, the solution turns light yellow-green. Remove excess carbon disulfide. Add sodium hypochlorite at 25-40°C for oxidation and take samples. Inspect the end point, filter, wash with water until neutral, and dry to get the finished product.
Purpose
1. Used as seed fungicides, insecticides, and fungicides in agriculture; used as lubricant additives in industry. This product is a super vulcanization accelerator for natural rubber, synthetic rubber and latex. It is often used together with thiazole accelerators and can also be used together with other accelerators as an accelerator for continuous rubber materials. This product decomposes slowly above 100℃ and precipitates free sulfur, so it can be used as a vulcanizing agent. The aging and heat resistance of the product are excellent. Mainly used for manufacturing tires, inner tubes, rubber shoes, medical supplies, cables and industrial products. Especially suitable for transparent, white and colored rubber industrial products. Used as vulcanization retardant in neoprene rubber.
2.It is a broad-spectrum protective fungicide with a residual effect period of about 7 days. It is mainly used to treat seeds and soil, prevent and control powdery mildew, smut, and rice seedling blight of cereal crops. It can also be used for some fruit tree and vegetable diseases. If 50% wettable powder is used for seed dressing, it can prevent and control rice blast, rice and flax leaf spot, and barley and wheat smut.
3.Used as an accelerator for adhesives such as nitrile rubber.
4. Antibacterial agents and deodorants used in soaps. Agricultural pesticides.
