Thiophenol
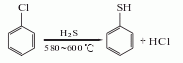
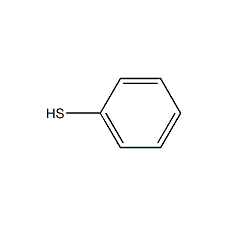
Structural formula
| Business number | 02YD |
|---|---|
| Molecular formula | C6H6S |
| Molecular weight | 110.18 |
| label |
phenylthiol, Benzene(yl)mercaptan, thiophenol, thiophenol, mercaptobenzene, Thiophenol (0311 restricted for export), Mercaptophenol, Mercaptobenzene, Fema 3616, Benzenethiol, Akos Bbs-00004420, Benzenethiolnylchloride, Mercapto-benzen, Phenol, thio-, Phenylthiol, aromatic sulfur compounds |
Numbering system
CAS number:108-98-5
MDL number:MFCD00004826
EINECS number:203-635-3
RTECS number:DC0525000
BRN number:506523
PubChem number:24901811
Physical property data
1. Properties: Colorless liquid with a suffocating odor. [1]
2. Melting point (℃): -14.8[2]
3. Boiling point (℃): 168.3[3]
4. Relative density (water = 1): 1.0728[4]
5. Relative vapor Density (air=1): 3.8[5]
6. Saturated vapor pressure (kPa): 0.186 (20℃)[6]
7. Critical pressure (MPa): 4.74[7]
8. Octanol/water partition coefficient: 2.52[8]
9. Flash point (℃): 51[9]
10. Explosion limit (%): No data yet
11. Lower explosion limit (%): 1.2[10]
12. Solubility: insoluble in water, miscible in ethanol, ether, benzene, and carbon disulfide. [11]
Toxicological data
1. Acute toxicity[12]
LD50: 46.2mg/kg (rat oral); 267mg/kg (mouse Oral); 300mg/kg (rat transdermal); 134mg/kg (rabbit transdermal)
LC50: 149mg/m3; 33ppm (rat inhalation, 4h )
2. Irritation No information available
Ecological data
1. Ecotoxicity[13]
LC100: 5mg/L (5h) (bluegill sunfish); 5mg/L ( 23h) (goldfish)
2. Biodegradability [14] Activated sludge method, 6d, BOD removal rate 30%~42% .
3. Non-biodegradability[15] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 34 hours (theoretical).
4. Bioaccumulation[16] BCF is 17 (theoretical).
Molecular structure data
1. Molar refractive index: 34.42
2. Molar volume (cm3/mol): 102.0
3. Isotonic specific volume (90.2K ): 255.7
4. Surface tension (dyne/cm): 39.4
5. Polarizability (10-24cm3): 13.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 46.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Toxic, flammable and corrosive. It has a strong irritating effect on the eyes, mucous membranes, respiratory tract and skin. Inhalation can cause larynx and bronchospasm, edema, chemical pneumonia, and pulmonary edema, resulting in death. LD5046.2mg/kg (rat oral). Production should be strictly enclosed, provide adequate local exhaust, and be as mechanized and automated as possible. Gas masks should be worn when contact with steam is possible, and operators should wear protective equipment.
2. Stability[17] Stable
3. Incompatible substances[18] Strong oxidizing agent, strong alkali
4. Polymerization hazard[19] No polymerization
Storage method
Storage Precautions[20] Storage in a cool, well-ventilated special warehouse, and implement the “two people to receive and receive, two people to keep” system. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Thiophenol can be obtained by reducing benzenesulfonic acid or benzenesulfonyl chloride with sulfuric acid and zinc, or by electrolytic reduction. Another preparation method uses chlorobenzene as raw material and reacts with hydrogen sulfide. Mix chlorobenzene and hydrogen sulfide at a molar ratio of 1:2 and react at 580-600°C. The conversion rate is more than 30%, and the yield of thiophenol is more than 70%.
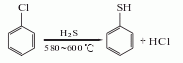
Purpose
1. Used as an intermediate for fine chemicals such as dyes, medicines, pesticides, polymerization inhibitors, antioxidants, etc., for example, used in the production of drugs thiamphenicol, fungicides, Kewen powder, etc.
2. Change the alkene from Z to E. Its sodium salt is weaker in alkalinity than sodium methoxide or sodium ethoxide, has strong nucleophilicity, and can selectively remove the methyl or benzyl groups of the quaternary iron salt.
3. Used in organic synthesis, pharmaceutical industry and as analytical reagents. [21]
