Zinc dibutyldithiocarbamate
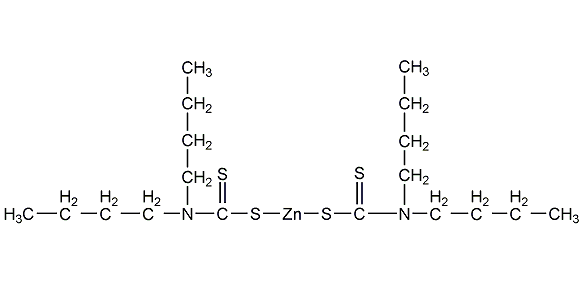

Structural formula
| Business number | 03QQ |
|---|---|
| Molecular formula | C18H36N2S4Zn |
| Molecular weight | 474.12 |
| label |
ZBC, Rubber accelerator BZ, Rubber accelerator ZDBC, accelerator, catalyst, Auxiliary |
Numbering system
CAS number:136-23-2
MDL number:MFCD00067274
EINECS number:205-232-8
RTECS number:ZH0175000
BRN number:None
PubChem ID:None
Physical property data
None
Toxicological data
Acute toxicity data :
Mouse abdominal cavity LD50:100mg/kg
Oncogenic data :
Mouse Sutra PortTDLo:290 gm/kg/78W-I
Mouse subcutaneous TDLo:100mg/kg
Ecological data
None
Molecular structure data
None
Compute chemical data
1. HydrophobicParameter calculation reference value (XlogP):
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 12
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA):6.5
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 112
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 3
Properties and stability
None
Storage method
Synthesis method
2. Preparation or source: by the action of butanediamine, carbon disulfide and sodium hydroxide After forming dibutyldithiocarbamic acid, it is prepared by reacting with zinc chloride
Purpose
is an effective peroxide decomposer and is often used together with the main antioxidant. Used in polypropylene and polyvinyl chloride. It is also used in rubber as rubber adhesive and latex curing accelerator. Surface charge: 0
9. Complexity: 112
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 3
Properties and stability
None
Storage method
Synthesis method
2. Preparation or source: by the action of butanediamine, carbon disulfide and sodium hydroxide After forming dibutyldithiocarbamic acid, it is prepared by reacting with zinc chloride
Purpose
is an effective peroxide decomposer and is often used together with the main antioxidant. Used in polypropylene and polyvinyl chloride. It is also used in rubber as rubber adhesive and latex curing accelerator.
le=”COLOR: #333333; mso-bidi-font-size: 10.5pt”>
